Mechanobiology of TGFβ signaling in the skeleton
- PMID: 26877077
- PMCID: PMC4875828
- DOI: 10.1016/j.matbio.2016.02.002
Mechanobiology of TGFβ signaling in the skeleton
Abstract
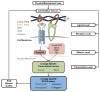
Physical and biochemical cues play fundamental roles in the skeleton at both the tissue and cellular levels. The precise coordination of these cues is essential for skeletal development and homeostasis, and disruption of this coordination can drive disease progression. The growth factor TGFβ is involved in both the regulation of and cellular response to the physical microenvironment. It is essential to summarize the current findings regarding the mechanisms by which skeletal cells integrate physical and biochemical cues so that we can identify and address remaining gaps that could ultimately improve skeletal health. In this review, we describe the role of TGFβ in mechanobiological signaling in bone and cartilage at the tissue and cellular levels. We provide detail on how static and dynamic physical cues at the macro-level are transmitted to the micro-level, ultimately leading to regulation at each level of the TGFβ pathway and to cell differentiation. The continued integration of engineering and biological approaches is needed to answer many remaining questions, such as the mechanisms by which cells generate a coordinated response to physical and biochemical cues. We propose one such mechanism, through which the combination of TGFβ and an optimal physical microenvironment leads to synergistic induction of downstream TGFβ signaling.
Copyright © 2016 International Society of Matrix Biology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

