Mechanisms of xenobiotic receptor activation: Direct vs. indirect
- PMID: 26877237
- PMCID: PMC4975672
- DOI: 10.1016/j.bbagrm.2016.02.006
Mechanisms of xenobiotic receptor activation: Direct vs. indirect
Abstract
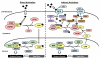
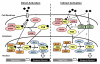
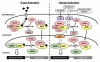
The so-called xenobiotic receptors (XRs) have functionally evolved into cellular sensors for both endogenous and exogenous stimuli by regulating the transcription of genes encoding drug-metabolizing enzymes and transporters, as well as those involving energy homeostasis, cell proliferation, and/or immune responses. Unlike prototypical steroid hormone receptors, XRs are activated through both direct ligand-binding and ligand-independent (indirect) mechanisms by a plethora of structurally unrelated chemicals. This review covers research literature that discusses direct vs. indirect activation of XRs. A particular focus is centered on the signaling control of the constitutive androstane receptor (CAR), the pregnane X receptor (PXR), and the aryl hydrocarbon receptor (AhR). We expect that this review will shed light on both the common and distinct mechanisms associated with activation of these three XRs. This article is part of a Special Issue entitled: Xenobiotic nuclear receptors: New Tricks for An Old Dog, edited by Dr. Wen Xie.
Keywords: Activation mechanism; AhR; CAR; PXR; Xenobiotic receptor.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
RNA-Seq reveals common and unique PXR- and CAR-target gene signatures in the mouse liver transcriptome.Biochim Biophys Acta. 2016 Sep;1859(9):1198-1217. doi: 10.1016/j.bbagrm.2016.04.010. Epub 2016 Apr 23. Biochim Biophys Acta. 2016. PMID: 27113289 Free PMC article.
-
Small-molecule modulators of PXR and CAR.Biochim Biophys Acta. 2016 Sep;1859(9):1141-1154. doi: 10.1016/j.bbagrm.2016.02.013. Epub 2016 Feb 24. Biochim Biophys Acta. 2016. PMID: 26921498 Free PMC article. Review.
-
Nuclear receptors and nonalcoholic fatty liver disease.Biochim Biophys Acta. 2016 Sep;1859(9):1083-1099. doi: 10.1016/j.bbagrm.2016.03.002. Epub 2016 Mar 4. Biochim Biophys Acta. 2016. PMID: 26962021 Free PMC article. Review.
-
Genomewide comparison of the inducible transcriptomes of nuclear receptors CAR, PXR and PPARα in primary human hepatocytes.Biochim Biophys Acta. 2016 Sep;1859(9):1218-1227. doi: 10.1016/j.bbagrm.2016.03.007. Epub 2016 Mar 17. Biochim Biophys Acta. 2016. PMID: 26994748
-
Regulation of drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR.Adv Drug Deliv Rev. 2010 Oct 30;62(13):1238-49. doi: 10.1016/j.addr.2010.08.006. Epub 2010 Aug 17. Adv Drug Deliv Rev. 2010. PMID: 20727377 Free PMC article. Review.
Cited by
-
Transcriptional Regulation of Drug Metabolizing CYP Enzymes by Proinflammatory Wnt5A Signaling in Human Coronary Artery Endothelial Cells.Front Pharmacol. 2021 May 17;12:619588. doi: 10.3389/fphar.2021.619588. eCollection 2021. Front Pharmacol. 2021. PMID: 34079452 Free PMC article.
-
Host-microbiota interactions: The aryl hydrocarbon receptor in the acute and chronic phases of cerebral ischemia.Front Immunol. 2022 Aug 12;13:967300. doi: 10.3389/fimmu.2022.967300. eCollection 2022. Front Immunol. 2022. PMID: 36032153 Free PMC article. Review.
-
Okadaic Acid Activates JAK/STAT Signaling to Affect Xenobiotic Metabolism in HepaRG Cells.Cells. 2023 Feb 28;12(5):770. doi: 10.3390/cells12050770. Cells. 2023. PMID: 36899906 Free PMC article.
-
Emerging roles of bile acids in mucosal immunity and inflammation.Mucosal Immunol. 2019 Jul;12(4):851-861. doi: 10.1038/s41385-019-0162-4. Epub 2019 Apr 5. Mucosal Immunol. 2019. PMID: 30952999 Review.
-
Enhanced DNA adduct formation by benzo[a]pyrene in human liver cells lacking cytochrome P450 oxidoreductase.Mutat Res Genet Toxicol Environ Mutagen. 2020 Apr;852:503162. doi: 10.1016/j.mrgentox.2020.503162. Epub 2020 Feb 22. Mutat Res Genet Toxicol Environ Mutagen. 2020. PMID: 32265041 Free PMC article.
References
-
- Forman BM, Evans RM. Nuclear hormone receptors activate direct, inverted, and everted repeats. Ann N Y Acad Sci. 1995;761:29–37. - PubMed
-
- Nagy L, Schwabe JWR. Mechanism of the nuclear receptor molecular switch. Trends in Biochemical Sciences. 2004;29:317–324. - PubMed
-
- McKenna NJ, Lanz RB, O’Malley BW. Nuclear Receptor Coregulators: Cellular and Molecular Biology. Endocrine Reviews. 1999;20:321–344. - PubMed
-
- Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

