A Systematic Review and Meta-Analysis of the Utility of Corticosteroids in the Treatment of Hyperemesis Gravidarum
- PMID: 26877629
- PMCID: PMC4745642
- DOI: 10.4137/NMI.S29532
A Systematic Review and Meta-Analysis of the Utility of Corticosteroids in the Treatment of Hyperemesis Gravidarum
Abstract
Background: Corticosteroids (CCS) are effective in reducing chemotherapy-induced nausea and vomiting, but it is unknown whether CCS are effective in treating hyperemesis gravidarum (HG).
Methods: We searched PubMed and ClinicalTrials.gov from inception to May 15, 2015, for randomized controlled trials examining the effects of CCS in HG.
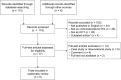
Results: We identified five trials (n = 310) examining the effects of CCS in women with HG. Meta-analysis was possible for one outcome (n = 214) and showed no significant effect of CCS on readmission rates (odds ratio, 0.37; 95% confidence internal: 0.1-1.35). Two small studies (n = 104) reported a reduction of vomiting episodes, and one (n = 24) found improvement of well-being, but no effect on other outcomes. None of the studies that investigated perinatal outcome (n = 173) found an effect of CCS and were underpowered to investigate teratogenic effects. We found evidence of publication bias.
Conclusion: Meta-analysis yielded no effect of CCS therapy on readmission rates. Single small studies indicated possible beneficial effects on other outcomes. Future high-quality trials are necessary and would benefit from consensus on HG definition and core outcomes of HG therapy.
Keywords: corticosteroids; effectiveness; hyperemesis gravidarum; treatment.
Figures

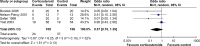

Similar articles
-
Variation in hyperemesis gravidarum definition and outcome reporting in randomised clinical trials: a systematic review.BJOG. 2018 Nov;125(12):1514-1521. doi: 10.1111/1471-0528.15272. Epub 2018 Jul 2. BJOG. 2018. PMID: 29727913
-
Burden, risk factors and outcomes of hyperemesis gravidarum in low-income and middle-income countries (LMICs): systematic review and meta-analysis protocol.BMJ Open. 2019 Apr 4;9(4):e025841. doi: 10.1136/bmjopen-2018-025841. BMJ Open. 2019. PMID: 30948589 Free PMC article.
-
Early nasogastric tube feeding in optimising treatment for hyperemesis gravidarum: the MOTHER randomised controlled trial (Maternal and Offspring outcomes after Treatment of HyperEmesis by Refeeding).BMC Pregnancy Childbirth. 2016 Jan 27;16:22. doi: 10.1186/s12884-016-0815-1. BMC Pregnancy Childbirth. 2016. PMID: 26819104 Free PMC article. Clinical Trial.
-
Hospital admission for hyperemesis gravidarum: a nationwide study of occurrence, reoccurrence and risk factors among 8.2 million pregnancies.Hum Reprod. 2016 Aug;31(8):1675-84. doi: 10.1093/humrep/dew128. Epub 2016 May 31. Hum Reprod. 2016. PMID: 27251205
-
Effectiveness of ginger supplementation in alleviating hyperemesis gravidarum: a systematic review and meta-analysis.Am J Transl Res. 2025 Mar 15;17(3):1568-1579. doi: 10.62347/TXKV6669. eCollection 2025. Am J Transl Res. 2025. PMID: 40226035 Free PMC article. Review.
Cited by
-
Nausea and vomiting of pregnancy - What's new?Auton Neurosci. 2017 Jan;202:62-72. doi: 10.1016/j.autneu.2016.05.002. Epub 2016 May 13. Auton Neurosci. 2017. PMID: 27209471 Free PMC article. Review.
-
Barriers and Challenges in Hyperemesis Gravidarum Research.Nutr Metab Insights. 2016 Feb 14;8(Suppl 1):33-9. doi: 10.4137/NMI.S29523. eCollection 2015. Nutr Metab Insights. 2016. PMID: 26917969 Free PMC article. Review.
-
Hyperemesis in Pregnancy: Complications and Treatment.Med Sci (Basel). 2025 Aug 14;13(3):132. doi: 10.3390/medsci13030132. Med Sci (Basel). 2025. PMID: 40843754 Free PMC article. Review.
-
Life-threatening complications of hyperemesis gravidarum.Exp Ther Med. 2021 Jun;21(6):642. doi: 10.3892/etm.2021.10074. Epub 2021 Apr 16. Exp Ther Med. 2021. PMID: 33968173 Free PMC article.
-
Systematic evidence map of evidence addressing the top 10 priority research questions for hyperemesis gravidarum.BMJ Open. 2022 Sep 6;12(9):e052687. doi: 10.1136/bmjopen-2021-052687. BMJ Open. 2022. PMID: 36691124 Free PMC article.
References
-
- Niebyl JR. Clinical practice. Nausea and vomiting in pregnancy. N Engl J Med. 2010;363(16):1544–1550. - PubMed
-
- Roseboom TJ, Ravelli AC, van der Post JA, et al. Maternal characteristics largely explain poor pregnancy outcome after hyperemesis gravidarum. Eur J Obstet Gynecol Reprod Biol. 2011;156(1):56–59. - PubMed
-
- Gazmararian JA, Petersen R, Jamieson DJ, et al. Hospitalizations during pregnancy among managed care enrollees. Obstet Gynecol. 2002;100(1):94–100. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

