Palladium-Catalyzed Directed C(sp3)-H Arylation of Saturated Heterocycles at C-3 Using a Concise Optimization Approach
- PMID: 26877706
- PMCID: PMC4736452
- DOI: 10.1002/ejoc.201501300
Palladium-Catalyzed Directed C(sp3)-H Arylation of Saturated Heterocycles at C-3 Using a Concise Optimization Approach
Abstract
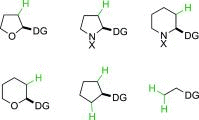
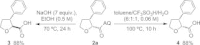
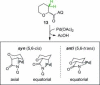
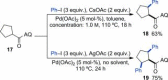
Saturated heterocycles, such as THFs, pyrrolidines, piperidines and THPs, are essential components of many biologically active compounds. Examples of C-H functionalization on these important ring systems remain scarce, especially at unactivated positions. Here we report the development of conditions for the palladium-catalyzed stereoselective C(sp3)-H arylation at unactivated 3-positions of 5- and 6-membered N- and O-heterocycles with aminoquinoline directing groups. Subtle differences in substrate structures altered their reactivity significantly; and different conditions were required to achieve high yields in each case. Successful conditions were developed using a short empirical optimization approach to cover reaction space with a limited set of variables. Excellent cis-selectivity was achieved in all cases, except for the THP substrate where minor trans-products were formed through a different palladacyclic intermediate. Here, differences in reactivity and selectivity with other directing groups were examined.
Keywords: C–H arylation; Homogeneous catalysis; N Heterocycles; O Heterocycles; Palladium.
Figures










References
-
- None
-
- Lorente A. , Lamariano‐Merketegi J. , Albericio F. , Álvarez M. , Chem. Rev. 2013. , 113 , 4567 –4610 . - PubMed
-
- Saleem M. , Kim H. J. , Ali M. S. , Lee Y. S. , Nat. Prod. Rep. 2005. , 22 , 696 –716 . - PubMed
-
- Galliford C. V. , Scheidt K. A. , Angew. Chem. Int. Ed. 2007. , 46 , 8748 –8758 ; Angew. Chem. 2007 , 119 , 8902 . - PubMed
-
- Felpin F.‐X. , Lebreton J. , Eur. J. Org. Chem. 2003. , 3693 –3712 .
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
