Endosulfan Induces CYP1A1 Expression Mediated through Aryl Hydrocarbon Receptor Signal Transduction by Protein Kinase C
- PMID: 26877836
- PMCID: PMC4751443
- DOI: 10.5487/TR.2015.31.4.339
Endosulfan Induces CYP1A1 Expression Mediated through Aryl Hydrocarbon Receptor Signal Transduction by Protein Kinase C
Abstract
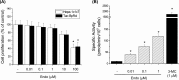
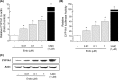
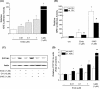
CYP1A1 is a phase I xenobiotic-metabolizing enzyme whose expression is mainly driven by AhR. Endosulfan is an organochlorine pesticide used agriculturally for a wide range of crops. In this study, we investigated the effect of endosulfan on CYP1A1 expression and regulation. Endosulfan significantly increased CYP1A1 enzyme activity as well as mRNA and protein levels. In addition, endosulfan markedly induced XRE transcriptional activity. CH-223191, an AhR antagonist, blocked the endosulfan-induced increase in CYP1A1 mRNA and protein expression. Moreover, endosulfan did not induce CYP1A1 gene expression in AhR-deficient mutant cells. Furthermore, endosulfan enhanced the phosphorylation of calcium calmodulin (CaM)-dependent protein kinase (CaMK) and protein kinase C (PKC). In conclusion, endosulfan-induced up-regulation of CYP1A1 is associated with AhR activation, which may be mediated by PKC-dependent pathways.
Keywords: Aryl hydrocarbon receptor; CYP1A1; Calcium; Endosulfan; Protein kinase C.
Figures

Similar articles
-
Up-regulation of CYP1A1 by rutaecarpine is dependent on aryl hydrocarbon receptor and calcium.Toxicology. 2009 Dec 21;266(1-3):38-47. doi: 10.1016/j.tox.2009.10.013. Epub 2009 Oct 21. Toxicology. 2009. PMID: 19853001
-
The role of protein kinase C in regulation of TCDD-mediated CYP1A1 gene expression.Toxicol Sci. 2005 Sep;87(1):27-37. doi: 10.1093/toxsci/kfi220. Epub 2005 Jun 9. Toxicol Sci. 2005. PMID: 15947024
-
Genotoxic polycyclic aromatic hydrocarbon ortho-quinones generated by aldo-keto reductases induce CYP1A1 via nuclear translocation of the aryl hydrocarbon receptor.Cancer Res. 2000 Feb 15;60(4):908-15. Cancer Res. 2000. PMID: 10706104
-
Ligand-independent activation of aryl hydrocarbon receptor signaling in PCB3-quinone treated HaCaT human keratinocytes.Toxicol Lett. 2015 Mar 18;233(3):258-66. doi: 10.1016/j.toxlet.2015.02.005. Epub 2015 Feb 7. Toxicol Lett. 2015. PMID: 25668756 Free PMC article.
-
Aryl hydrocarbon receptor mediates laminar fluid shear stress-induced CYP1A1 activation and cell cycle arrest in vascular endothelial cells.Cardiovasc Res. 2008 Mar 1;77(4):809-18. doi: 10.1093/cvr/cvm095. Epub 2007 Dec 7. Cardiovasc Res. 2008. PMID: 18065768
Cited by
-
Protective effects of Camellia japonica flower extract against urban air pollutants.BMC Complement Altern Med. 2019 Jan 28;19(1):30. doi: 10.1186/s12906-018-2405-4. BMC Complement Altern Med. 2019. PMID: 30691451 Free PMC article.
-
Synergistic cellular effects including mitochondrial destabilization, autophagy and apoptosis following low-level exposure to a mixture of lipophilic persistent organic pollutants.Sci Rep. 2017 Jul 5;7(1):4728. doi: 10.1038/s41598-017-04654-0. Sci Rep. 2017. PMID: 28680151 Free PMC article.
-
Isolevuglandins and cardiovascular disease.Prostaglandins Other Lipid Mediat. 2018 Nov;139:29-35. doi: 10.1016/j.prostaglandins.2018.10.002. Epub 2018 Oct 5. Prostaglandins Other Lipid Mediat. 2018. PMID: 30296489 Free PMC article. Review.
-
Endocrine disruptors, aryl hydrocarbon receptor and cortisol secretion.J Endocrinol Invest. 2024 Oct;47(10):2407-2419. doi: 10.1007/s40618-024-02371-w. Epub 2024 Apr 18. J Endocrinol Invest. 2024. PMID: 38637430 Free PMC article. Review.
-
Curcumin, a Multifaceted Hormetic Agent, Mediates an Intricate Crosstalk between Mitochondrial Turnover, Autophagy, and Apoptosis.Oxid Med Cell Longev. 2020 Jul 18;2020:3656419. doi: 10.1155/2020/3656419. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32765806 Free PMC article. Review.
References
-
- Nelson D.R., Koymans L., Kamataki T., Stegeman J.J., Feyereisen R., Waxman D.J., Waterman M.R., Gotoh O., Coon M.J., Estabrook R.W., Gunsalus I.C., Nebert D.W. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics. (1996);6:1–42. doi: 10.1097/00008571-199602000-00002. - DOI - PubMed
-
- Dipple A. Reactions of polycyclic aromatic hydrocarbons with DNA. IARC Sci. Publ. (1994);125:107–129. - PubMed
-
- Perdew G.H. Association of the Ah receptor with the 90-kDa heat shock protein. J. Biol. Chem. (1988);263:13802–13805. - PubMed
LinkOut - more resources
Full Text Sources

