Antioxidant Activity and Anti-wrinkle Effects of Aceriphyllum rossii Leaf Ethanol Extract
- PMID: 26877839
- PMCID: PMC4751446
- DOI: 10.5487/TR.2015.31.4.363
Antioxidant Activity and Anti-wrinkle Effects of Aceriphyllum rossii Leaf Ethanol Extract
Abstract
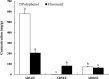
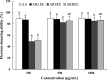
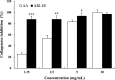
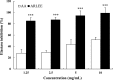
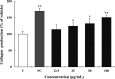
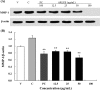
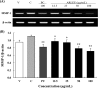
We evaluated the antioxidant activity and anti-wrinkle effects of Aceriphyllum rossii leaf ethanol extract (ARLEE) in vitro using human dermal fibroblasts. The total polyphenol and flavonoid contents of ARLEE were 578.6 and 206.3 mg/g, respectively. At a concentration of 250 μg/mL, the electron-donating ability of ARLEE was 87.1%. In comparison with the vehicle, ARLEE treatment at 100 μg/mL significantly increased type I procollagen synthesis (p < 0.01) by 50.7%. In vitro ARLEE treatment (10 mg/mL) inhibited collagenase and elastase activity by 97.1% and 99.2%, respectively. Compared with the control, ascorbic acid treatment at 100 μg/mL significantly decreased matrix metalloproteinase (MMP)-1 protein expression (p < 0.01) by 37.0%. ARLEE treatment at 50 μg/mL significantly decreased MMP-1 protein expression (p < 0.01) by 46.1%. Ascorbic acid and ARLEE treatments at 100 μg/mL significantly decreased MMP-1 mRNA expression (p < 0.01) by 26.1% and 36.1%, respectively. From these results, we conclude that ARLEE has excellent antioxidant activity and even better anti-wrinkle effects than ascorbic acid in human dermal fibroblasts. These results suggest that ARLEE could be used in functional cosmetics for the prevention or alleviation of skin wrinkles induced by ultraviolet rays.
Keywords: Aceriphyllum rossii; Antioxidant activity; Collagenase activity; MMP-1; Type I procollagen.
Figures
Similar articles
-
Anti-Photoaging Effects of Angelica acutiloba Root Ethanol Extract in Human Dermal Fibroblasts.Toxicol Res. 2017 Apr;33(2):125-134. doi: 10.5487/TR.2017.33.2.125. Epub 2017 Apr 15. Toxicol Res. 2017. PMID: 28503261 Free PMC article.
-
Evaluation of Skin Anti-aging Potential of Citrus reticulata Blanco Peel.Pharmacognosy Res. 2016 Jul-Sep;8(3):160-8. doi: 10.4103/0974-8490.182913. Pharmacognosy Res. 2016. PMID: 27365982 Free PMC article.
-
Amentoflavone-Enriched Selaginella rossii Protects against Ultraviolet- and Oxidative Stress-Induced Aging in Skin Cells.Life (Basel). 2022 Dec 14;12(12):2106. doi: 10.3390/life12122106. Life (Basel). 2022. PMID: 36556471 Free PMC article.
-
Action mechanism of anti-wrinkle effect of Rhamnus yoshinoi methanol extract in human dermal fibroblast and keratinocyte cell lines.Toxicol Res. 2019 Dec 4;36(1):69-77. doi: 10.1007/s43188-019-00007-3. eCollection 2020 Jan. Toxicol Res. 2019. PMID: 32042713 Free PMC article.
-
Recent advances in characterizing biological mechanisms underlying UV-induced wrinkles: a pivotal role of fibrobrast-derived elastase.Arch Dermatol Res. 2008 Apr;300 Suppl 1:S7-20. doi: 10.1007/s00403-007-0798-x. Arch Dermatol Res. 2008. PMID: 17968573 Review.
Cited by
-
Antiphotoaging properties of Zingiber montanum essential oil isolated by solvent-free microwave extraction against ultraviolet B-irradiated human dermal fibroblasts.Toxicol Res. 2021 Oct 3;38(2):235-248. doi: 10.1007/s43188-021-00107-z. eCollection 2022 Apr. Toxicol Res. 2021. PMID: 35419276 Free PMC article.
-
Anti-Inflammatory, Barrier-Protective, and Antiwrinkle Properties of Agastache rugosa Kuntze in Human Epidermal Keratinocytes.Biomed Res Int. 2020 Oct 26;2020:1759067. doi: 10.1155/2020/1759067. eCollection 2020. Biomed Res Int. 2020. PMID: 33195687 Free PMC article.
-
Biological Activity and Component Analyses of Chamaecyparis obtusa Leaf Extract: Evaluation of Antiwrinkle and Cell Protection Effects in UVA-Irradiated Cells.Medicina (Kaunas). 2023 Apr 13;59(4):755. doi: 10.3390/medicina59040755. Medicina (Kaunas). 2023. PMID: 37109713 Free PMC article.
-
3,5,6,7,8,3',4'-Heptamethoxyflavone, a Citrus Flavonoid, Inhibits Collagenase Activity and Induces Type I Procollagen Synthesis in HDFn Cells.Int J Mol Sci. 2018 Feb 22;19(2):620. doi: 10.3390/ijms19020620. Int J Mol Sci. 2018. PMID: 29470423 Free PMC article.
-
Galangin Activates the ERK/AKT-Driven Nrf2 Signaling Pathway to Increase the Level of Reduced Glutathione in Human Keratinocytes.Biomol Ther (Seoul). 2017 Jul 1;25(4):427-433. doi: 10.4062/biomolther.2016.112. Biomol Ther (Seoul). 2017. PMID: 27829272 Free PMC article.
References
-
- Welgus H.G., Campbell E.J., Cury J.D., Eisen A.Z., Senior R.M., Wilhelm S.M., Goldberg G.I. Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J. Clin. Invest. (1990);86:1496–1502. doi: 10.1172/JCI114867. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

