Measles virus nucleocapsid protein increases osteoblast differentiation in Paget's disease
- PMID: 26878170
- PMCID: PMC4767344
- DOI: 10.1172/JCI82012
Measles virus nucleocapsid protein increases osteoblast differentiation in Paget's disease
Abstract
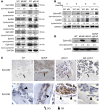
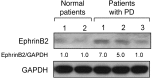
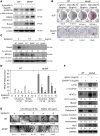
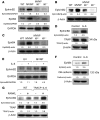
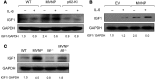
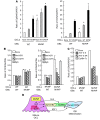
Paget's disease (PD) is characterized by focal and dramatic bone resorption and formation. Treatments that target osteoclasts (OCLs) block both pagetic bone resorption and formation; therefore, PD offers key insights into mechanisms that couple bone resorption and formation. Here, we evaluated OCLs from 3 patients with PD and determined that measles virus nucleocapsid protein (MVNP) was expressed in 70% of these OCLs. Moreover, transgenic mice with OCL-specific expression of MVNP (MVNP mice) developed PD-like bone lesions that required MVNP-dependent induction of high IL-6 expression levels in OCLs. In contrast, mice harboring a knockin of p62P394L (p62-KI mice), which is the most frequent PD-associated mutation, exhibited increased bone resorption, but not formation. Evaluation of OCLs from MVNP, p62-KI, and WT mice revealed increased IGF1 expression in MVNP-expressing OCLs that resulted from the high IL-6 expression levels in these cells. IL-6, in turn, increased the expression of coupling factors, specifically ephrinB2 on OCLs and EphB4 on osteoblasts (OBs). IGF1 enhanced ephrinB2 expression on OCLs and OB differentiation. Importantly, ephrinB2 and IGF1 levels were increased in MVNP-expressing OCLs from patients with PD and MVNP-transduced human OCLs compared with levels detected in controls. Further, anti-IGF1 or anti-IGF1R blocked Runx2 and osteocalcin upregulation in OBs cocultured with MVNP-expressing OCLs. These results suggest that in PD, MVNP upregulates IL-6 and IGF1 in OCLs to increase ephrinB2-EphB4 coupling and bone formation.
Figures

References
-
- Basle M, Minard MF, Rebel A. [Structure and ultrastructure of osteoblasts and of the osteoid tissue in Paget’s disease of bone (author’s transl)] Pathol Biol (Paris) 1978;26(8):475–479. - PubMed
-
- Zajac AJ, Phillips PE. Paget’s disease of bone: clinical features and treatment. Clin Exp Rheumatol. 1985;3(1):75–88. - PubMed
-
- Rebel A, Malkani K, Basle M, Bregeon C. Osteoclast ultrastructure in Paget’s disease. Calcif Tissue Res. 1976;(2):187–199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

