Using MD Simulations To Calculate How Solvents Modulate Solubility
- PMID: 26878198
- PMCID: PMC4945102
- DOI: 10.1021/acs.jctc.5b00934
Using MD Simulations To Calculate How Solvents Modulate Solubility
Abstract
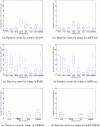
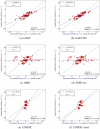
Here, our interest is in predicting solubility in general, and we focus particularly on predicting how the solubility of particular solutes is modulated by the solvent environment. Solubility in general is extremely important, both for theoretical reasons - it provides an important probe of the balance between solute-solute and solute-solvent interactions - and for more practical reasons, such as how to control the solubility of a given solute via modulation of its environment, as in process chemistry and separations. Here, we study how the change of solvent affects the solubility of a given compound. That is, we calculate relative solubilities. We use MD simulations to calculate relative solubility and compare our calculated values with experiment as well as with results from several other methods, SMD and UNIFAC, the latter of which is commonly used in chemical engineering design. We find that straightforward solubility calculations based on molecular simulations using a general small-molecule force field outperform SMD and UNIFAC both in terms of accuracy and coverage of the relevant chemical space.
Figures


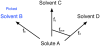
Similar articles
-
Comparative assessment of physics-based in silico methods to calculate relative solubilities.J Comput Aided Mol Des. 2024 Oct 29;38(1):36. doi: 10.1007/s10822-024-00576-y. J Comput Aided Mol Des. 2024. PMID: 39470860
-
How hydrophobic hydration responds to solute size and attractions: Theory and simulations.J Chem Phys. 2009 Sep 21;131(11):115102. doi: 10.1063/1.3227031. J Chem Phys. 2009. PMID: 19778151
-
Extended Hansen solubility approach: naphthalene in individual solvents.J Pharm Sci. 1981 Nov;70(11):1260-4. doi: 10.1002/jps.2600701120. J Pharm Sci. 1981. PMID: 7299673
-
Volumetric measurements in binary solvents: theory to experiment.Biophys Chem. 2011 Jun;156(1):3-12. doi: 10.1016/j.bpc.2010.12.004. Epub 2010 Dec 24. Biophys Chem. 2011. PMID: 21236557 Review.
-
The hydrophobic effect. 2. Relative importance of the hydrophobic effect on the solubility of hydrophobes and pharmaceuticals in H-bonded solvents.J Pharm Sci. 1998 Aug;87(8):998-1014. doi: 10.1021/js9702980. J Pharm Sci. 1998. PMID: 9687345 Review.
Cited by
-
Evaluation of nine condensed-phase force fields of the GROMOS, CHARMM, OPLS, AMBER, and OpenFF families against experimental cross-solvation free energies.Phys Chem Chem Phys. 2021 Jun 21;23(23):13055-13074. doi: 10.1039/d1cp00215e. Epub 2021 Jun 8. Phys Chem Chem Phys. 2021. PMID: 34105547 Free PMC article.
-
Computer aided greenness by design approach for resolving and quantifying Triamterene and Hydrochlorothiazide in pharmaceutical mixtures employing multiscale dynamics simulations.Sci Rep. 2025 Aug 6;15(1):28668. doi: 10.1038/s41598-025-13486-2. Sci Rep. 2025. PMID: 40764789 Free PMC article.
-
Computational prediction of drug solubility in water-based systems: Qualitative and quantitative approaches used in the current drug discovery and development setting.Int J Pharm. 2018 Apr 5;540(1-2):185-193. doi: 10.1016/j.ijpharm.2018.01.044. Epub 2018 Feb 6. Int J Pharm. 2018. PMID: 29421301 Free PMC article. Review.
-
Comparative assessment of physics-based in silico methods to calculate relative solubilities.J Comput Aided Mol Des. 2024 Oct 29;38(1):36. doi: 10.1007/s10822-024-00576-y. J Comput Aided Mol Des. 2024. PMID: 39470860
-
Infinite Dilution Activity Coefficients as Constraints for Force Field Parametrization and Method Development.J Chem Theory Comput. 2019 May 14;15(5):3066-3074. doi: 10.1021/acs.jctc.8b01029. Epub 2019 Apr 15. J Chem Theory Comput. 2019. PMID: 30939010 Free PMC article.
References
-
- Hopfinger AJ, Esposito EX, Llinaàs A, Glen RC, Goodman JM. Findings of the Challenge To Predict Aqueous Solubility. J. Chem. Inf. Model. 2009;49:1–5. - PubMed
-
- Fredenslund A, Jones RL, Prausnitz JM. Group-contribution estimation of activity coefficients in nonideal liquid mixtures. AIChE J. 1975;21:1086–1099.
-
- Hansen HK, Rasmussen P, Fredenslund A, Schiller M, Gmehling J. Vapor-liquid equilibria by UNIFAC group contribution. 5. Revision and extension. Ind. Eng. Chem. Res. 1991;30:2352–2355.
-
- Wittig R, Lohmann J, Gmehling J. VaporLiquid Equilibria by UNIFAC Group Contribution. 6. Revision and Extension. Ind. Eng. Chem. Res. 2003;42:183–188.
-
- Gmehling J, Li J, Schiller M. A modified UNIFAC model. 2. Present parameter matrix and results for different thermodynamic properties. Ind. Eng. Chem. Res. 1993;32:178–193.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
