Short-term consumption of n-3 PUFAs increases murine IL-5 levels, but IL-5 is not the mechanistic link between n-3 fatty acids and changes in B-cell populations
- PMID: 26878780
- PMCID: PMC4757814
- DOI: 10.1016/j.jnutbio.2015.09.012
Short-term consumption of n-3 PUFAs increases murine IL-5 levels, but IL-5 is not the mechanistic link between n-3 fatty acids and changes in B-cell populations
Abstract
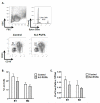
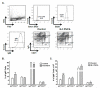
N-3 polyunsaturated fatty acids (PUFAs) exert immunomodulatory effects on B cells. We previously demonstrated that n-3 PUFAs enhanced the relative percentage and/or frequency of select B2 cell subsets. The objectives here were to determine if n-3 PUFAs (a) could boost cytokines that target B-cell frequency, (b) enhance the frequency of the B1 population and (c) to identify the mechanism by which n-3 PUFAs modify the proportion of B cells. Administration of n-3 PUFAs as fish oil to C57BL/6 mice enhanced secretion of the Th2 cytokine IL-5 but not IL-9 or IL-13. N-3 PUFAs had no influence on the percentage or frequency of peritoneal B1 or B2 cells. Subsequent experiments with IL-5(-/-) knockout mice showed n-3 PUFAs decreased the percentage of bone marrow B220(lo)IgM(hi) cells and increased the proportion and number of splenic IgM(+)IgD(lo)CD21(lo) cells compared to the control. These results, when compared with our previous findings with wild-type mice, suggested IL-5 had no role in mediating the effect of n-3 PUFAs on B-cell populations. To confirm this conclusion, we assayed IL-5 secretion in a diet-induced obesity model in which n-3 PUFAs enhanced the frequency of select B-cell subsets. N-3 PUFA supplementation as ethyl esters to obesogenic diets did not alter circulating IL-5 levels. Altogether, the data establish that n-3 PUFAs as fish oil can increase circulating IL-5 in lean mice, which has implications for several disease end points, but this increase in IL-5 is not the mechanistic link between n-3 PUFAs and changes in B-cell populations.
Keywords: B cells; DHA; EPA; IL-5; n-3 PUFAs.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
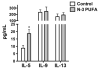
Similar articles
-
Dietary fatty acids influence the production of Th1- but not Th2-type cytokines.J Leukoc Biol. 2001 Mar;69(3):449-57. J Leukoc Biol. 2001. PMID: 11261793
-
n-3 PUFAs enhance the frequency of murine B-cell subsets and restore the impairment of antibody production to a T-independent antigen in obesity.J Lipid Res. 2013 Nov;54(11):3130-8. doi: 10.1194/jlr.M042457. Epub 2013 Aug 28. J Lipid Res. 2013. PMID: 23986558 Free PMC article.
-
Dietary omega-3 fatty acids enhance the B1 but not the B2 cell immune response in mice with antigen-induced peritonitis.J Nutr Biochem. 2014 Feb;25(2):111-7. doi: 10.1016/j.jnutbio.2013.09.010. Epub 2013 Oct 30. J Nutr Biochem. 2014. PMID: 24332949
-
Effects of altering dietary fatty acid composition on prostaglandin synthesis and fertility.Prostaglandins Leukot Essent Fatty Acids. 1999 Nov;61(5):275-87. doi: 10.1054/plef.1999.0101. Prostaglandins Leukot Essent Fatty Acids. 1999. PMID: 10670689 Review.
-
N-3 polyunsaturated fatty acids modulate B cell activity in pre-clinical models: Implications for the immune response to infections.Eur J Pharmacol. 2016 Aug 15;785:10-17. doi: 10.1016/j.ejphar.2015.03.100. Epub 2015 May 27. Eur J Pharmacol. 2016. PMID: 26022530 Free PMC article. Review.
Cited by
-
Effects of Omega-3 Fatty Acids on Immune Cells.Int J Mol Sci. 2019 Oct 11;20(20):5028. doi: 10.3390/ijms20205028. Int J Mol Sci. 2019. PMID: 31614433 Free PMC article. Review.
-
Key roles for lipid mediators in the adaptive immune response.J Clin Invest. 2018 Jul 2;128(7):2724-2731. doi: 10.1172/JCI97951. Epub 2018 Jul 2. J Clin Invest. 2018. PMID: 30108196 Free PMC article. Review.
-
A diet rich in omega-3 fatty acid improves periodontitis and tissue destruction by MMP2- and MMP9-linked inflammation in a murine model.Odontology. 2024 Jan;112(1):185-199. doi: 10.1007/s10266-023-00831-y. Epub 2023 Jun 28. Odontology. 2024. PMID: 37378834 Free PMC article.
-
Separate and combined effects of advanced age and obesity on mammary adipose inflammation, immunosuppression and tumor progression in mouse models of triple negative breast cancer.Front Oncol. 2023 Jan 4;12:1031174. doi: 10.3389/fonc.2022.1031174. eCollection 2022. Front Oncol. 2023. PMID: 36686775 Free PMC article.
-
Determining the effect of different cooking methods on the nutritional composition of salmon (Salmo salar) and chilean jack mackerel (Trachurus murphyi) fillets.PLoS One. 2017 Jul 7;12(7):e0180993. doi: 10.1371/journal.pone.0180993. eCollection 2017. PLoS One. 2017. PMID: 28686742 Free PMC article.
References
-
- Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2015;1851:469–84. - PubMed
-
- Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergology International. 2015;64:27–34. - PubMed
-
- Skulas-Ray AC. Omega-3 fatty acids and inflammation: A perspective on the challenges of evaluating efficacy in clinical research. Prostaglandins & Other Lipid Mediators. 2015;116-117C:104–11. - PubMed
-
- Miles EA, Calder PC. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. The British Journal of Nutrition. 2012;107(Suppl 2):S171–84. - PubMed
-
- Nigam A, Talajic M, Roy D, Nattel S, Lambert J, Nozza A, et al. Fish oil for the reduction of atrial fibrillation recurrence, inflammation, and oxidative stress. Journal of the American College of Cardiology. 2014;64:1441–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

