LGI1 acts presynaptically to regulate excitatory synaptic transmission during early postnatal development
- PMID: 26878798
- PMCID: PMC4754946
- DOI: 10.1038/srep21769
LGI1 acts presynaptically to regulate excitatory synaptic transmission during early postnatal development
Abstract
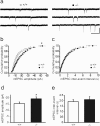
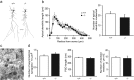
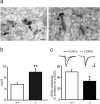
The secreted leucine-rich glioma inactivated 1 (LGI1) protein is an important actor for human seizures of both genetic and autoimmune etiology: mutations in LGI1 cause inherited temporal lobe epilepsy, while LGI1 is involved in antibody-mediated encephalitis. Remarkably, Lgi1-deficient (Lgi1(-/-)) mice recapitulate the epileptic disorder and display early-onset spontaneous seizures. To understand how Lgi1-deficiency leads to seizures during postnatal development, we here investigated the early functional and structural defects occurring before seizure onset in Lgi1(-/-) mice. We found an increased excitatory synaptic transmission in hippocampal slices from Lgi1(-/-) mice. No structural alteration in the morphology of pyramidal cell dendrites and synapses was observed at this stage, indicating that Lgi1-deficiency is unlikely to trigger early developmental abnormalities. Consistent with the presynaptic subcellular localization of the protein, Lgi1-deficiency caused presynaptic defects, with no alteration in postsynaptic AMPA receptor activity in Lgi1-/- pyramidal cells before seizure onset. Presynaptic dysfunction led to increased synaptic glutamate levels, which were associated with hyperexcitable neuronal networks. Altogether, these data show that Lgi1 acts presynaptically as a negative modulator of excitatory synaptic transmission during early postnatal development. We therefore here reveal that increased presynaptic glutamate release is a key early event resulting from Lgi1-deficiency, which likely contributes to epileptogenesis.
Figures


References
-
- Morante-Redolat J. M. et al. Mutations in the LGI1/Epitempin gene on 10q24 cause autosomal dominant lateral temporal epilepsy. Hum. Mol. Genet. 11, 1119–1128 (2002). - PubMed
-
- Michelucci R., Pasini E. & Nobile C. Lateral temporal lobe epilepsies: clinical and genetic features. Epilepsia 50 Suppl 5, 52–54 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

