Quantitative molecular phenotyping with topically applied SERS nanoparticles for intraoperative guidance of breast cancer lumpectomy
- PMID: 26878888
- PMCID: PMC4754709
- DOI: 10.1038/srep21242
Quantitative molecular phenotyping with topically applied SERS nanoparticles for intraoperative guidance of breast cancer lumpectomy
Abstract
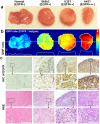
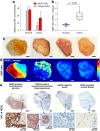
There is a need to image excised tissues during tumor-resection procedures in order to identify residual tumors at the margins and to guide their complete removal. The imaging of dysregulated cell-surface receptors is a potential means of identifying the presence of diseases with high sensitivity and specificity. However, due to heterogeneities in the expression of protein biomarkers in tumors, molecular-imaging technologies should ideally be capable of visualizing a multiplexed panel of cancer biomarkers. Here, we demonstrate that the topical application and quantification of a multiplexed cocktail of receptor-targeted surface-enhanced Raman scattering (SERS) nanoparticles (NPs) enables rapid quantitative molecular phenotyping (QMP) of the surface of freshly excised tissues to determine the presence of disease. In order to mitigate the ambiguity due to nonspecific sources of contrast such as off-target binding or uneven delivery, a ratiometric method is employed to quantify the specific vs. nonspecific binding of the multiplexed NPs. Validation experiments with human tumor cell lines, fresh human tumor xenografts in mice, and fresh human breast specimens demonstrate that QMP imaging of excised tissues agrees with flow cytometry and immunohistochemistry, and that this technique may be achieved in less than 15 minutes for potential intraoperative use in guiding breast-conserving surgeries.
Conflict of interest statement
Dr. Liu has an issued patent: “Raman imaging devices and methods of molecular imaging.” The other authors declare no competing financial interests.
Figures



References
-
- Fisher B. et al. Twenty-Year Follow-up of a Randomized Trial Comparing Total Mastectomy, Lumpectomy, and Lumpectomy plus Irradiation for the Treatment of Invasive Breast Cancer. New Engl. J. Med. 347, 1233–1241 (2002). - PubMed
-
- DeSantis C., Ma J., Bryan L. & Jemal A. Breast cancer statistics, 2013. CA-Cancer J. Clin. 64, 52–62 (2014). - PubMed
-
- Waljee J. F., Hu E. S., Newman L. A. & Alderman A. K. Predictors of re-excision among women undergoing breast-conserving surgery for cancer. Ann. Surg. Oncol. 15, 1297–1303 (2008). - PubMed
-
- Moran M. S. et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer.Int. J. Radiat. Oncol. 88, 553–564 (2014). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

