Upregulation of inducible NO synthase by exogenous adenosine in vascular smooth muscle cells activated by inflammatory stimuli in experimental diabetes
- PMID: 26879172
- PMCID: PMC4754884
- DOI: 10.1186/s12933-016-0349-x
Upregulation of inducible NO synthase by exogenous adenosine in vascular smooth muscle cells activated by inflammatory stimuli in experimental diabetes
Abstract
Background: Adenosine has been shown to induce nitric oxide (NO) production via inducible NO synthase (iNOS) activation in vascular smooth muscle cells (VSMCs). Although this is interpreted as a beneficial vasodilating pathway in vaso-occlusive disorders, iNOS is also involved in diabetic vascular dysfunction. Because the turnover of and the potential to modulate iNOS by adenosine in experimental diabetes have not been explored, we hypothesized that both the adenosine system and control of iNOS function are impaired in VSMCs from streptozotocin-diabetic rats.
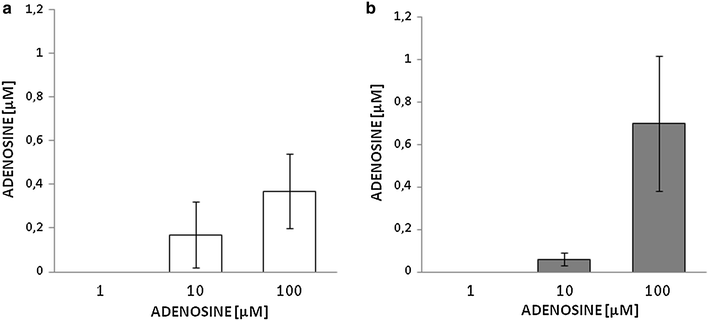
Methods: Male Sprague-Dawley rats were injected with streptozotocin once to induce diabetes. Aortic VSMCs from diabetic and nondiabetic rats were isolated, cultured and exposed to lipopolysaccharide (LPS) plus a cytokine mix for 24 h in the presence or absence of (1) exogenous adenosine and related compounds, and/or (2) pharmacological agents affecting adenosine turnover. iNOS functional expression was determined by immunoblotting and NO metabolite assays. Concentrations of adenosine, related compounds and metabolites thereof were assayed by HPLC. Vasomotor responses to adenosine were determined in endothelium-deprived aortic rings.
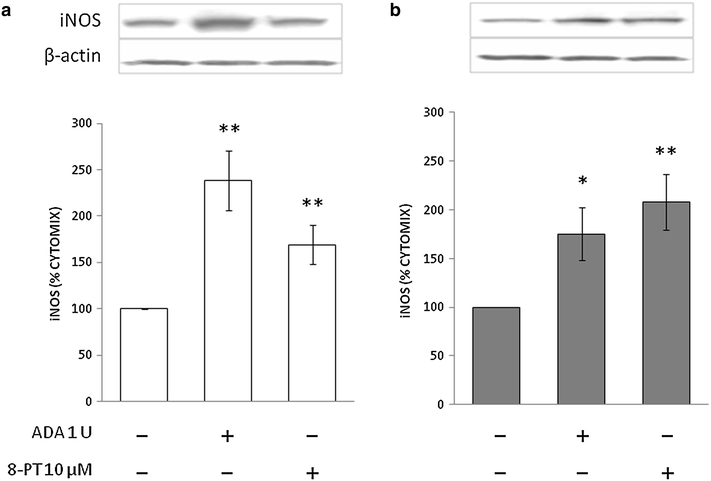
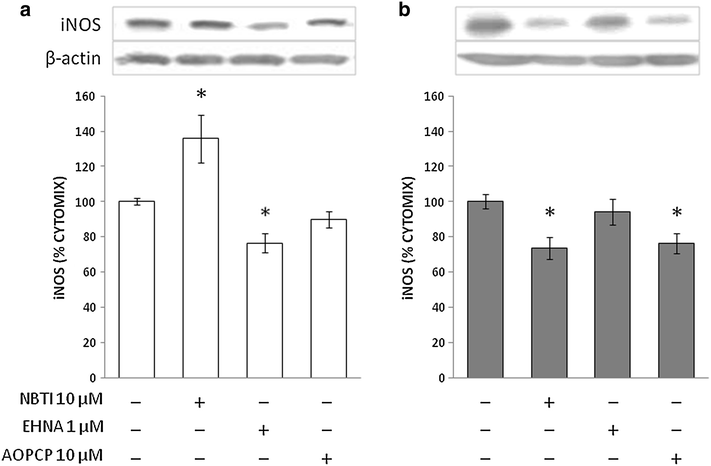
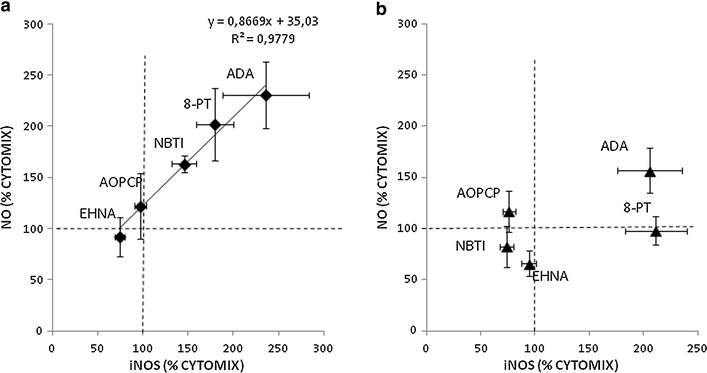
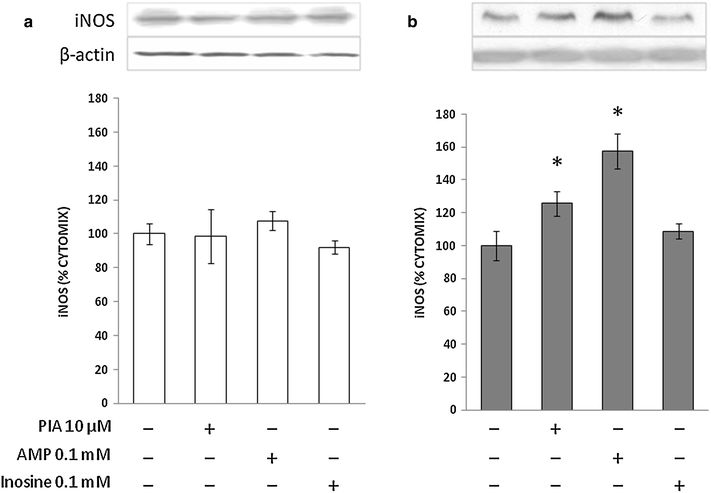
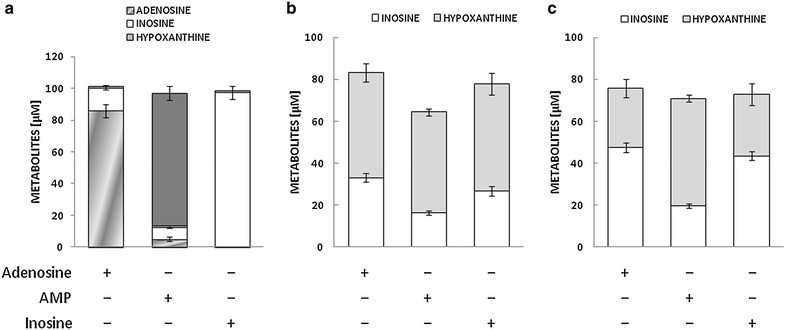
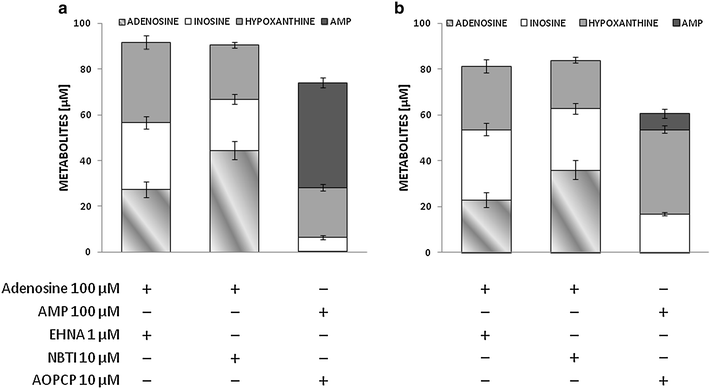
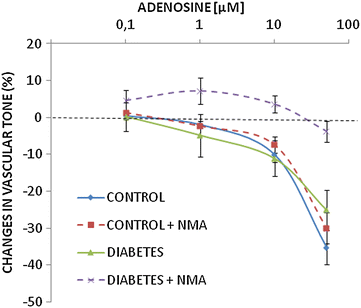
Results: Treatment with adenosine-degrading enzymes or receptor antagonists increased iNOS formation in activated VSMCs from nondiabetic and diabetic rats. Following treatment with the adenosine transport inhibitor NBTI, iNOS levels increased in nondiabetic but decreased in diabetic VSMCs. The amount of secreted NO metabolites was uncoupled from iNOS levels in diabetic VSMCs. Addition of high concentrations of adenosine and its precursors or analogues enhanced iNOS formation solely in diabetic VSMCs. Exogenous adenosine and AMP were completely removed from the culture medium and converted into metabolites. A tendency towards elevated inosine generation was observed in diabetic VSMCs, which were also less sensitive to CD73 inhibition, but inosine supplementation did not affect iNOS levels. Pharmacological inhibition of NOS abolished adenosine-induced vasorelaxation in aortic tissues from diabetic but not nondiabetic animals.
Conclusions: Endogenous adenosine prevented cytokine- and LPS-induced iNOS activation in VSMCs. By contrast, supplementation with adenosine and its precursors or analogues enhanced iNOS levels in diabetic VSMCs. This effect was associated with alterations in exogenous adenosine turnover. Thus, overactivation of the adenosine system may foster iNOS-mediated diabetic vascular dysfunction.
Figures
Similar articles
-
Diabetes undermines estrogen control of inducible nitric oxide synthase function in rat aortic smooth muscle cells through overexpression of estrogen receptor-beta.Circulation. 2003 Jul 15;108(2):211-7. doi: 10.1161/01.CIR.0000079311.39939.94. Epub 2003 Jun 23. Circulation. 2003. PMID: 12821541
-
Protein kinase C and protein tyrosine kinase mediate lipopolysaccharide- and cytokine-induced nitric oxide formation in vascular smooth muscle cells of rats.Sheng Li Xue Bao. 2003 Jun 25;55(3):265-72. Sheng Li Xue Bao. 2003. PMID: 12817292
-
Increased iNOS activity in vascular smooth muscle cells from diabetic rats: potential role of Ca(2+)/calmodulin-dependent protein kinase II delta 2 (CaMKIIδ(2)).Atherosclerosis. 2013 Jan;226(1):88-94. doi: 10.1016/j.atherosclerosis.2012.10.062. Epub 2012 Nov 8. Atherosclerosis. 2013. PMID: 23177014
-
[Analysis of Vascular Cell Response to Hypertension Induced by Pressure Loading and Its Application as a Tool for Exploring Pharmacological Modes of Action].Yakugaku Zasshi. 2016;136(11):1485-1490. doi: 10.1248/yakushi.16-00188. Yakugaku Zasshi. 2016. PMID: 27803479 Review. Japanese.
-
[Regulation of the expression of inducible nitric oxide synthase by prostanoids].Yakugaku Zasshi. 2009 Oct;129(10):1211-4. doi: 10.1248/yakushi.129.1211. Yakugaku Zasshi. 2009. PMID: 19797875 Review. Japanese.
Cited by
-
Sex Dependence in Control of Renal Haemodynamics and Excretion in Streptozotocin Diabetic Rats-Role of Adenosine System and Nitric Oxide.Int J Mol Sci. 2024 Jul 13;25(14):7699. doi: 10.3390/ijms25147699. Int J Mol Sci. 2024. PMID: 39062939 Free PMC article.
-
Identification and quantification of protein nitration sites in human coronary artery smooth muscle cells in the absence and presence of peroxynitrous acid/peroxynitrite.Redox Biol. 2023 Aug;64:102799. doi: 10.1016/j.redox.2023.102799. Epub 2023 Jun 26. Redox Biol. 2023. PMID: 37413764 Free PMC article.
-
Transcriptomic effects of adenosine 2A receptor deletion in healthy and endotoxemic murine myocardium.Purinergic Signal. 2017 Mar;13(1):27-49. doi: 10.1007/s11302-016-9536-1. Epub 2016 Sep 30. Purinergic Signal. 2017. PMID: 27696085 Free PMC article.
-
Advanced glycation end-products decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells.Cardiovasc Diabetol. 2017 Apr 20;16(1):52. doi: 10.1186/s12933-017-0531-9. Cardiovasc Diabetol. 2017. PMID: 28427390 Free PMC article.
-
Alteration of purinergic signaling in diabetes: Focus on vascular function.J Mol Cell Cardiol. 2020 Mar;140:1-9. doi: 10.1016/j.yjmcc.2020.02.004. Epub 2020 Feb 11. J Mol Cell Cardiol. 2020. PMID: 32057736 Free PMC article. Review.
References
-
- Antonioli L, Blandizzi C, Csóka B, Pacher P, Haskó G. Adenosine signalling in diabetes mellitus—pathophysiology and therapeutic considerations. Nat Rev Endocrinol. 2015;11:228–241. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

