Investigation of the therapeutic potential of N-acetyl cysteine and the tools used to define nigrostriatal degeneration in vivo
- PMID: 26879220
- PMCID: PMC4792766
- DOI: 10.1016/j.taap.2016.02.010
Investigation of the therapeutic potential of N-acetyl cysteine and the tools used to define nigrostriatal degeneration in vivo
Abstract
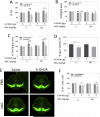
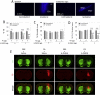
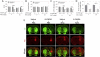
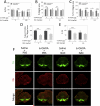
The glutathione precursor N-acetyl-L-cysteine (NAC) is currently being tested on Parkinson's patients for its neuroprotective properties. Our studies have shown that NAC can elicit protection in glutathione-independent manners in vitro. Thus, the goal of the present study was to establish an animal model of NAC-mediated protection in which to dissect the underlying mechanism. Mice were infused intrastriatally with the oxidative neurotoxicant 6-hydroxydopamine (6-OHDA; 4 μg) and administered NAC intraperitoneally (100mg/kg). NAC-treated animals exhibited higher levels of the dopaminergic terminal marker tyrosine hydroxylase (TH) in the striatum 10d after 6-OHDA. As TH expression is subject to stress-induced modulation, we infused the tracer FluoroGold into the striatum to retrogradely label nigrostriatal projection neurons. As expected, nigral FluoroGold staining and cell counts of FluoroGold(+) profiles were both more sensitive measures of nigrostriatal degeneration than measurements relying on TH alone. However, NAC failed to protect dopaminergic neurons 3 weeks following 6-OHDA, an effect verified by four measures: striatal TH levels, nigral TH levels, nigral TH(+) cell counts, and nigral FluoroGold levels. Some degree of mild toxicity of FluoroGold and NAC was evident, suggesting that caution must be exercised when relying on FluoroGold as a neuron-counting tool and when designing experiments with long-term delivery of NAC--such as clinical trials on patients with chronic disorders. Finally, the strengths and limitations of the tools used to define nigrostriatal degeneration are discussed.
Keywords: Dopamine; FluoroGold; N-acetylcysteine; Neurodegeneration; Parkinson's disease; Retrograde; The reference trap.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


References
-
- Adair JC, Knoefel JE, Morgan N. Controlled trial of N-acetylcysteine for patients with probable Alzheimer's disease. Neurology. 2001;57:1515–1517. - PubMed
-
- Algren DA. Review of N-acetyl cysteine for the treatment of acetaminophen (paracetamol) toxicity in pediatrics.. World Health Organization: Second Meeting of the Subcommittee of the Expert Committee on the Selection and Use of Essential Medicines; Geneva, Switzerland. 2008. [12/10/15]. Webpage: http://www.who.int/selection_medicines/committees/subcommittee/2/acetylc....
-
- Aluf Y, Vaya J, Khatib S, Loboda Y, Kizhner S, Finberg JP. Specific oxidative stress profile associated with partial striatal dopaminergic depletion by 6-hydroxydopamine as assessed by a novel multifunctional marker molecule. Free Radic. Res. 2010;44:635–644. - PubMed
-
- Ataei S, Hadjibabaie M, Moslehi A, Taghizadeh-Ghehi M, Ashouri A, Amini E, Gholami K, Hayatshahi A, Vaezi M, Ghavamzadeh A. A double-blind, randomized, controlled trial on N-acetylcysteine for the prevention of acute kidney injury in patients undergoing allogeneic hematopoietic stem cell transplantation. Hematol. Oncol. 2015;33:67–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

