Selective Targeting of a Novel Epsin-VEGFR2 Interaction Promotes VEGF-Mediated Angiogenesis
- PMID: 26879230
- PMCID: PMC4798882
- DOI: 10.1161/CIRCRESAHA.115.307679
Selective Targeting of a Novel Epsin-VEGFR2 Interaction Promotes VEGF-Mediated Angiogenesis
Abstract
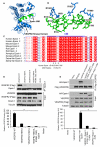
Rationale: We previously reported that vascular endothelial growth factor (VEGF)-induced binding of VEGF receptor 2 (VEGFR2) to epsins 1 and 2 triggers VEGFR2 degradation and attenuates VEGF signaling. The epsin ubiquitin interacting motif (UIM) was shown to be required for the interaction with VEGFR2. However, the molecular determinants that govern how epsin specifically interacts with and regulates VEGFR2 were unknown.
Objective: The goals for the present study were as follows: (1) to identify critical molecular determinants that drive the specificity of the epsin and VEGFR2 interaction and (2) to ascertain whether such determinants were critical for physiological angiogenesis in vivo.
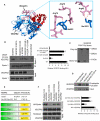
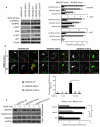
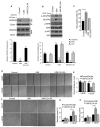
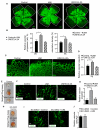
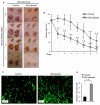
Methods and results: Structural modeling uncovered 2 novel binding surfaces within VEGFR2 that mediate specific interactions with epsin UIM. Three glutamic acid residues in epsin UIM were found to interact with residues in VEGFR2. Furthermore, we found that the VEGF-induced VEGFR2-epsin interaction promoted casitas B-lineage lymphoma-mediated ubiquitination of epsin, and uncovered a previously unappreciated ubiquitin-binding surface within VEGFR2. Mutational analysis revealed that the VEGFR2-epsin interaction is supported by VEGFR2 interacting specifically with the UIM and with ubiquitinated epsin. An epsin UIM peptide, but not a mutant UIM peptide, potentiated endothelial cell proliferation, migration and angiogenic properties in vitro, increased postnatal retinal angiogenesis, and enhanced VEGF-induced physiological angiogenesis and wound healing.
Conclusions: Distinct residues in the epsin UIM and VEGFR2 mediate specific interactions between epsin and VEGFR2, in addition to UIM recognition of ubiquitin moieties on VEGFR2. These novel interactions are critical for pathophysiological angiogenesis, suggesting that these sites could be selectively targeted by therapeutics to modulate angiogenesis.
Keywords: VEGFR2 protein, mouse; epsin; neovascularization, physiologic; ubiquitin; ubiquitination.
© 2016 American Heart Association, Inc.
Figures

References
-
- Tille JC, Wang X, Lipson KE, McMahon G, Ferrara N, Zhu Z, Hicklin DJ, Sleeman JP, Eriksson U, Alitalo K, Pepper MS. Vascular endothelial growth factor (VEGF) receptor-2 signaling mediates VEGF-C(deltaNdeltaC)- and VEGF-A-induced angiogenesis in vitro. Experimental cell research. 2003;285:286–98. - PubMed
-
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–71. - PubMed
-
- Duval M, Bedard-Goulet S, Delisle C, Gratton JP. Vascular endothelial growth factor-dependent down-regulation of Flk-1/KDR involves Cbl-mediated ubiquitination. Consequences on nitric oxide production from endothelial cells. The Journal of biological chemistry. 2003;278:20091–7. - PubMed
-
- Weinstein BM, Lawson ND. Arteries, veins, Notch, and VEGF. Cold Spring Harbor symposia on quantitative biology. 2002;67:155–62. - PubMed
-
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature medicine. 2003;9:669–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 HL121954/HL/NHLBI NIH HHS/United States
- R01 EY017017/EY/NEI NIH HHS/United States
- 1F32HL121954-01/HL/NHLBI NIH HHS/United States
- R01 HL137229/HL/NHLBI NIH HHS/United States
- F31 HL127982/HL/NHLBI NIH HHS/United States
- P20 RR018758/RR/NCRR NIH HHS/United States
- R01 HL093242/HL/NHLBI NIH HHS/United States
- P01 HL085607/HL/NHLBI NIH HHS/United States
- 1F31HL127982-01/HL/NHLBI NIH HHS/United States
- R01 HL118676/HL/NHLBI NIH HHS/United States
- U54 HD090255/HD/NICHD NIH HHS/United States
- R01 HL130845/HL/NHLBI NIH HHS/United States
- R01HL-130845/HL/NHLBI NIH HHS/United States
- R01HL-118676/HL/NHLBI NIH HHS/United States
- R01HL-093242/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

