Expression of Kir4.1 and Kir5.1 inwardly rectifying potassium channels in oligodendrocytes, the myelinating cells of the CNS
- PMID: 26879293
- PMCID: PMC5225165
- DOI: 10.1007/s00429-016-1199-8
Expression of Kir4.1 and Kir5.1 inwardly rectifying potassium channels in oligodendrocytes, the myelinating cells of the CNS
Abstract
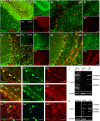
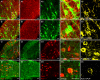
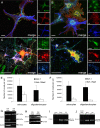
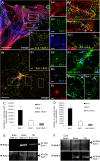
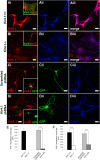
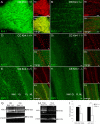
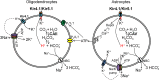
The inwardly rectifying K+ channel subtype Kir5.1 is only functional as a heteromeric channel with Kir4.1. In the CNS, Kir4.1 is localised to astrocytes and is the molecular basis of their strongly negative membrane potential. Oligodendrocytes are the specialised myelinating glia of the CNS and their resting membrane potential provides the driving force for ion and water transport that is essential for myelination. However, little is known about the ion channel profile of mature myelinating oligodendrocytes. Here, we identify for the first time colocalization of Kir5.1 with Kir4.1 in oligodendrocytes in white matter. Immunolocalization with membrane-bound Na+/K+-ATPase and western blot of the plasma membrane fraction of the optic nerve, a typical CNS white matter tract containing axons and the oligodendrocytes that myelinate them, demonstrates that Kir4.1 and Kir5.1 are colocalized on oligodendrocyte cell membranes. Co-immunoprecipitation provides evidence that oligodendrocytes and astrocytes express a combination of homomeric Kir4.1 and heteromeric Kir4.1/Kir5.1 channels. Genetic knock-out and shRNA to ablate Kir4.1 indicates plasmalemmal expression of Kir5.1 in glia is largely dependent on Kir4.1 and the plasmalemmal anchoring protein PSD-95. The results demonstrate that, in addition to astrocytes, oligodendrocytes express both homomeric Kir4.1 and heteromeric Kir4.1/Kir5.1 channels. In astrocytes, these channels are essential to their key functions of K+ uptake and CO2/H+ chemosensation. We propose Kir4.1/Kir5.1 channels have equivalent functions in oligodendrocytes, maintaining myelin integrity in the face of large ionic shifts associated with action potential propagation along myelinated axons.
Keywords: Astrocyte; Glia; Inward rectifying potassium channel; Oligodendrocyte; Potassium regulation; White matter.
Conflict of interest statement
The authors declare that they have no conflicts of interest. Informed consent All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted. Animal rights All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

