Hybrid modeling as a QbD/PAT tool in process development: an industrial E. coli case study
- PMID: 26879643
- PMCID: PMC4839057
- DOI: 10.1007/s00449-016-1557-1
Hybrid modeling as a QbD/PAT tool in process development: an industrial E. coli case study
Abstract
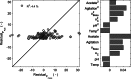
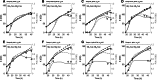
Process understanding is emphasized in the process analytical technology initiative and the quality by design paradigm to be essential for manufacturing of biopharmaceutical products with consistent high quality. A typical approach to developing a process understanding is applying a combination of design of experiments with statistical data analysis. Hybrid semi-parametric modeling is investigated as an alternative method to pure statistical data analysis. The hybrid model framework provides flexibility to select model complexity based on available data and knowledge. Here, a parametric dynamic bioreactor model is integrated with a nonparametric artificial neural network that describes biomass and product formation rates as function of varied fed-batch fermentation conditions for high cell density heterologous protein production with E. coli. Our model can accurately describe biomass growth and product formation across variations in induction temperature, pH and feed rates. The model indicates that while product expression rate is a function of early induction phase conditions, it is negatively impacted as productivity increases. This could correspond with physiological changes due to cytoplasmic product accumulation. Due to the dynamic nature of the model, rational process timing decisions can be made and the impact of temporal variations in process parameters on product formation and process performance can be assessed, which is central for process understanding.
Keywords: Dynamic modeling; E. coli; High cell density fermentation; Hybrid modeling; Upstream bioprocess development/optimization.
Figures



References
-
- Fuhr T, Holcomb M, Rutten P (2009) Why quality-by-design should be on the executive team's agenda: developing new strategies for new times. In: Losch M, Schrader U (eds) Outpacing change in pharma operations. McKinsey & Co, pp 195–203
-
- FDA (2004) Guidance for industry-PAT—a framework for innovative pharmaceutical development, manufacturing, and quality assurance. Pharmaceutical CGMPs
-
- Chew W, Sharratt P. Trends in process analytical technology. Anal Methods. 2010;2(10):1412–1438. doi: 10.1039/c0ay00257g. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

