Non-canonical features of the Golgi apparatus in bipolar epithelial neural stem cells
- PMID: 26879757
- PMCID: PMC4754753
- DOI: 10.1038/srep21206
Non-canonical features of the Golgi apparatus in bipolar epithelial neural stem cells
Abstract
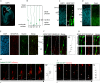
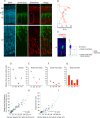
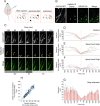
Apical radial glia (aRG), the stem cells in developing neocortex, are unique bipolar epithelial cells, extending an apical process to the ventricle and a basal process to the basal lamina. Here, we report novel features of the Golgi apparatus, a central organelle for cell polarity, in mouse aRGs. The Golgi was confined to the apical process but not associated with apical centrosome(s). In contrast, in aRG-derived, delaminating basal progenitors that lose apical polarity, the Golgi became pericentrosomal. The aRG Golgi underwent evolutionarily conserved, accordion-like compression and extension concomitant with cell cycle-dependent nuclear migration. Importantly, in line with endoplasmic reticulum but not Golgi being present in the aRG basal process, its plasma membrane contained glycans lacking Golgi processing, consistent with direct ER-to-cell surface membrane traffic. Our study reveals hitherto unknown complexity of neural stem cell polarity, differential Golgi contribution to their specific architecture, and fundamental Golgi re-organization upon cell fate change.
Figures

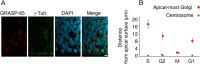





Similar articles
-
Lipidomic processes in homeostatic and LPS-modified cell renewal cycle. Role of phosphatidylinositol 3-kinase pathway in biomembrane synthesis and restitution of apical epithelial membrane.J Physiol Pharmacol. 2003 Dec;54(4):533-51. J Physiol Pharmacol. 2003. PMID: 14726609
-
Deciphering the Golgi apparatus: from imaging to genes.Traffic. 2008 Sep;9(10):1613-7. doi: 10.1111/j.1600-0854.2008.00769.x. Epub 2008 May 22. Traffic. 2008. PMID: 18503640
-
A secretory Golgi bypass route to the apical surface domain of epithelial MDCK cells.Traffic. 2009 Nov;10(11):1685-95. doi: 10.1111/j.1600-0854.2009.00984.x. Epub 2009 Sep 9. Traffic. 2009. PMID: 19765262
-
Formation and maintenance of the Golgi apparatus in plant cells.Int Rev Cell Mol Biol. 2014;310:221-87. doi: 10.1016/B978-0-12-800180-6.00006-2. Int Rev Cell Mol Biol. 2014. PMID: 24725428 Review.
-
Genesis of polarity in renal tubular cells.Miner Electrolyte Metab. 1986;12(1):20-4. Miner Electrolyte Metab. 1986. PMID: 3007959 Review.
Cited by
-
Regulation of Cell Delamination During Cortical Neurodevelopment and Implication for Brain Disorders.Front Neurosci. 2022 Feb 23;16:824802. doi: 10.3389/fnins.2022.824802. eCollection 2022. Front Neurosci. 2022. PMID: 35281509 Free PMC article. Review.
-
Breasi-CRISPR: an efficient genome-editing method to interrogate protein localization and protein-protein interactions in the embryonic mouse cortex.Development. 2022 Sep 15;149(18):dev200616. doi: 10.1242/dev.200616. Epub 2022 Sep 26. Development. 2022. PMID: 35993342 Free PMC article.
-
Roots of the Malformations of Cortical Development in the Cell Biology of Neural Progenitor Cells.Front Neurosci. 2022 Jan 5;15:817218. doi: 10.3389/fnins.2021.817218. eCollection 2021. Front Neurosci. 2022. PMID: 35069108 Free PMC article. Review.
-
Endogenous mutant Huntingtin alters the corticogenesis via lowering Golgi recruiting ARF1 in cortical organoid.Mol Psychiatry. 2024 Oct;29(10):3024-3039. doi: 10.1038/s41380-024-02562-0. Epub 2024 Apr 23. Mol Psychiatry. 2024. PMID: 38654124 Free PMC article.
-
Radial glia progenitor polarity in health and disease.Front Cell Dev Biol. 2024 Oct 2;12:1478283. doi: 10.3389/fcell.2024.1478283. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39416687 Free PMC article. Review.
References
-
- Götz M. & Huttner W. B. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 6, 777–788 (2005). - PubMed
-
- Taverna E., Götz M. & Huttner W. B. The cell biology of neurogenesis: toward an understanding of the development and evolution of the neocortex. Annu. Rev. Cell Dev. Biol. 30, 465–502 (2014). - PubMed
-
- Taverna E. & Huttner W. B. Neural progenitor nuclei IN Motion. Neuron 67, 906–914 (2010). - PubMed
-
- Lee H. O. & Norden C. Mechanisms controlling arrangements and movements of nuclei in pseudostratified epithelia. Trends Cell Biol 23, 141–50 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

