Heterotrimeric G protein signaling via GIV/Girdin: Breaking the rules of engagement, space, and time
- PMID: 26879989
- PMCID: PMC5123561
- DOI: 10.1002/bies.201500133
Heterotrimeric G protein signaling via GIV/Girdin: Breaking the rules of engagement, space, and time
Abstract
Canonical signal transduction via heterotrimeric G proteins is spatially and temporally restricted, that is, triggered exclusively at the plasma membrane (PM), only by agonist activation of G protein-coupled receptors (GPCRs) via a process that completes within a few hundred milliseconds. Recently, a rapidly emerging paradigm has revealed a non-canonical pathway for activation of heterotrimeric G proteins by the non-receptor guanidine-nucleotide exchange factor (GEF), GIV/Girdin. This pathway has distinctive temporal and spatial features and an unusual profile of receptor engagement: diverse classes of receptors, not just GPCRs can engage with GIV to trigger such activation. Such activation is spatially and temporally unrestricted, that is, can occur both at the PM and on internal membranes discontinuous with the PM, and can continue for prolonged periods of time. Here, we provide the most complete up-to-date review of the molecular mechanisms that govern the unique spatiotemporal aspects of non-canonical G protein activation by GIV and the relevance of this new paradigm in health and disease.
Keywords: Golgi; autophagy; cdk5: Girdin; growth factor receptor tyrosine kinases; heterotrimeric G proteins.
© 2016 WILEY Periodicals, Inc.
Figures
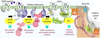
 ) by chance encounter with GEFs, which could be either GPCRs, i.e., receptor GEFs or non-receptor GEFs (such as GIV/Girdin). Such nucleotide exchange is inhibited (red
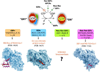
) by chance encounter with GEFs, which could be either GPCRs, i.e., receptor GEFs or non-receptor GEFs (such as GIV/Girdin). Such nucleotide exchange is inhibited (red  ) by GDIs. Once GTP-bound, Gα is active, dissociates from Gβγ-dimers until the GTP is hydrolyzed, inorganic phosphate (iP) is released and Gα returns to inactive state. This step of GTP hydrolysis is sped up by GAPs. Lower panel: The structural modules (red ribbons) which impart enzymatic properties to GAPs, GDIs and GEFs is shown in complex with Gα-subunit (in blue): (from left to right) GAPs accelerate GTP hydrolysis via a RGS box domain, as shown here in the case of RGS4 bound to an active conformation of Gαi1. GDIs inhibit nucleotide exchange via a Go-Loco/GPR domain, as shown here in the case of RGS14 bound to an inactive conformation of Gαi1. In the case of non-receptor GEFs, while some work via unknown module(s), a newly emerging subfamily uses a short stretch of amino acids to trigger nucleotide exchange using similar structural basis as shown here in the case of KB-752 synthetic peptide bound to an inactive conformation of Gαi1.
) by GDIs. Once GTP-bound, Gα is active, dissociates from Gβγ-dimers until the GTP is hydrolyzed, inorganic phosphate (iP) is released and Gα returns to inactive state. This step of GTP hydrolysis is sped up by GAPs. Lower panel: The structural modules (red ribbons) which impart enzymatic properties to GAPs, GDIs and GEFs is shown in complex with Gα-subunit (in blue): (from left to right) GAPs accelerate GTP hydrolysis via a RGS box domain, as shown here in the case of RGS4 bound to an active conformation of Gαi1. GDIs inhibit nucleotide exchange via a Go-Loco/GPR domain, as shown here in the case of RGS14 bound to an inactive conformation of Gαi1. In the case of non-receptor GEFs, while some work via unknown module(s), a newly emerging subfamily uses a short stretch of amino acids to trigger nucleotide exchange using similar structural basis as shown here in the case of KB-752 synthetic peptide bound to an inactive conformation of Gαi1.

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

