Three-dimensional Organotypic Culture Models of Human Hepatocellular Carcinoma
- PMID: 26880118
- PMCID: PMC4754778
- DOI: 10.1038/srep21174
Three-dimensional Organotypic Culture Models of Human Hepatocellular Carcinoma
Abstract
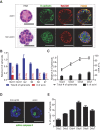
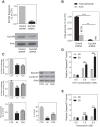
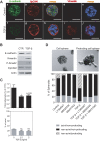
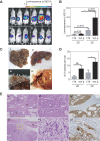
Three-dimensional cell culture methods are viable in vitro approaches that facilitate the examination of biological features cancer cells present in vivo. In this study, we demonstrate that hepatocellular carcinoma (HCC) cells in porous alginate scaffolds can generate organoid-like spheroids that mimic numerous features of glandular epithelium in vivo, such as acinar morphogenesis and apical expression patterns of EpCAM, a hepatic stem/progenitor cell marker highly expressed in a subset of HCC with stemness features. We show that the activation of Wnt/β-catenin signaling, an essential pathway for maintaining HCC stemness, is required for EpCAM(+) HCC spheroid formation as well as the maintenance of the acinous structure. Furthermore, we demonstrate that EpCAM(+) HCC cells cultured as spheroids are more sensitive to TGF/β-induced epithelial-mesenchymal transition with highly tumorigenic and metastatic potential in vivo compared to conventional two-dimensional (2D) culture. In addition, HCC cells in EpCAM(+) spheroids are more resistant to chemotherapeutic agents than 2D-cultured cells. The alginate scaffold-based organotypic culture system is a promising, reliable, and easy system that can be configured into a high throughput fashion for the identification of critical signaling pathways and screening of molecular drug targets specific for HCC.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Jemal A. et al. Global cancer statistics. CA Cancer J.Clin 61, 69–90 (2011). - PubMed
-
- Siegel R., Naishadham D. & Jemal A. Cancer statistics, 2013. CA Cancer J.Clin 63, 11–30 (2013). - PubMed
-
- Weinstein I. B. Cancer. Addiction to oncogenes–the Achilles heal of cancer. Science (New York, NY) 297, 63–64 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

