Neuroligin 1 regulates spines and synaptic plasticity via LIMK1/cofilin-mediated actin reorganization
- PMID: 26880202
- PMCID: PMC4754719
- DOI: 10.1083/jcb.201509023
Neuroligin 1 regulates spines and synaptic plasticity via LIMK1/cofilin-mediated actin reorganization
Abstract
Neuroligin (NLG) 1 is important for synapse development and function, but the underlying mechanisms remain unclear. It is known that at least some aspects of NLG1 function are independent of the presynaptic neurexin, suggesting that the C-terminal domain (CTD) of NLG1 may be sufficient for synaptic regulation. In addition, NLG1 is subjected to activity-dependent proteolytic cleavage, generating a cytosolic CTD fragment, but the significance of this process remains unknown. In this study, we show that the CTD of NLG1 is sufficient to (a) enhance spine and synapse number, (b) modulate synaptic plasticity, and (c) exert these effects via its interaction with spine-associated Rap guanosine triphosphatase-activating protein and subsequent activation of LIM-domain protein kinase 1/cofilin-mediated actin reorganization. Our results provide a novel postsynaptic mechanism by which NLG1 regulates synapse development and function.
© 2016 Liu et al.
Figures

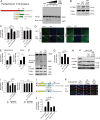
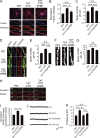


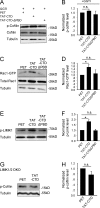

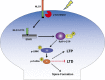
Comment in
-
Breaking down to build up: Neuroligin's C-terminal domain strengthens the synapse.J Cell Biol. 2016 Feb 15;212(4):375-7. doi: 10.1083/jcb.201601081. J Cell Biol. 2016. PMID: 26880198 Free PMC article.
Similar articles
-
Breaking down to build up: Neuroligin's C-terminal domain strengthens the synapse.J Cell Biol. 2016 Feb 15;212(4):375-7. doi: 10.1083/jcb.201601081. J Cell Biol. 2016. PMID: 26880198 Free PMC article.
-
Activity-dependent proteolytic cleavage of neuroligin-1.Neuron. 2012 Oct 18;76(2):410-22. doi: 10.1016/j.neuron.2012.10.003. Epub 2012 Oct 17. Neuron. 2012. PMID: 23083742
-
Transsynaptic signaling by activity-dependent cleavage of neuroligin-1.Neuron. 2012 Oct 18;76(2):396-409. doi: 10.1016/j.neuron.2012.07.006. Epub 2012 Oct 17. Neuron. 2012. PMID: 23083741 Free PMC article.
-
Role of LIMK1-cofilin-actin axis in dendritic spine dynamics in Alzheimer's disease.Cell Death Dis. 2025 Jun 3;16(1):431. doi: 10.1038/s41419-025-07741-7. Cell Death Dis. 2025. PMID: 40461464 Free PMC article. Review.
-
Regulation and signaling of the LIM domain kinases.Bioessays. 2025 Jan;47(1):e2400184. doi: 10.1002/bies.202400184. Epub 2024 Oct 3. Bioessays. 2025. PMID: 39361252 Review.
Cited by
-
DHCR24 exerts neuroprotection upon inflammation-induced neuronal death.J Neuroinflammation. 2017 Nov 7;14(1):215. doi: 10.1186/s12974-017-0991-6. J Neuroinflammation. 2017. PMID: 29115990 Free PMC article.
-
Long-term effects of stress early in life on microRNA-30a and its network: Preventive effects of lurasidone and potential implications for depression vulnerability.Neurobiol Stress. 2020 Nov 23;13:100271. doi: 10.1016/j.ynstr.2020.100271. eCollection 2020 Nov. Neurobiol Stress. 2020. PMID: 33344724 Free PMC article.
-
Annexin A6 mitigates neurological deficit in ischemia/reperfusion injury by promoting synaptic plasticity.CNS Neurosci Ther. 2024 Feb;30(2):e14639. doi: 10.1111/cns.14639. CNS Neurosci Ther. 2024. PMID: 38380783 Free PMC article.
-
Melatonin-Pretreated Mesenchymal Stem Cells Improved Cognition in a Diabetic Murine Model.Front Physiol. 2021 Mar 18;12:628107. doi: 10.3389/fphys.2021.628107. eCollection 2021. Front Physiol. 2021. PMID: 33815140 Free PMC article.
-
Neuroprotective Effects of Ginsenoside-Rg1 Against Depression-Like Behaviors via Suppressing Glial Activation, Synaptic Deficits, and Neuronal Apoptosis in Rats.Front Immunol. 2018 Dec 7;9:2889. doi: 10.3389/fimmu.2018.02889. eCollection 2018. Front Immunol. 2018. Retraction in: Front Immunol. 2024 Jun 03;15:1437785. doi: 10.3389/fimmu.2024.1437785. PMID: 30581440 Free PMC article. Retracted.
References
-
- Aizawa H., Wakatsuki S., Ishii A., Moriyama K., Sasaki Y., Ohashi K., Sekine-Aizawa Y., Sehara-Fujisawa A., Mizuno K., Goshima Y., and Yahara I.. 2001. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat. Neurosci. 4:367–373. 10.1038/86011 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

