Effect of TGF-β1 on the Migration and Recruitment of Mesenchymal Stem Cells after Vascular Balloon Injury: Involvement of Matrix Metalloproteinase-14
- PMID: 26880204
- PMCID: PMC4754777
- DOI: 10.1038/srep21176
Effect of TGF-β1 on the Migration and Recruitment of Mesenchymal Stem Cells after Vascular Balloon Injury: Involvement of Matrix Metalloproteinase-14
Abstract
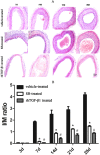
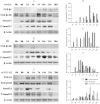
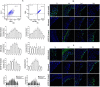
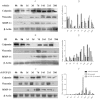
Restenosis or occlusion after vascular procedures is ascribed to intimal hyperplasia. Transforming growth factor (TGF)-β1 is involved in recruitment of mesenchymal stem cells (MSCs) following arterial injury, and its release from latent TGF-binding protein by matrix metalloproteinase (MMP)-14-induced proteolysis contributes to neointima formation. However, the relationship between MMP-14 and TGF-β1 activation in restenosis is unknown. This study investigated the relationship using a rat model of balloon-induced injury. Rats were assigned to vehicle-, SB431542 (SB)-, or recombinant human (rh)TGF-β1-treated groups and examined at various time points after balloon-induced injury for expression of TGF-β1/Smad signalling pathway components, MMP-14 and MSCs markers including Nestin, CD29, and Sca1(+)CD29(+)CD11b/c(-)CD45(-). Intimal hyperplasia was reduced in SB- and rhTGF-β1-treated rats. The expression of TGF-β1, TGF-β1RI, and Smad2/3 was decreased, but the levels of phosphorylated Smad2/3 were higher in SB-treated rats than vehicle-treated after 7 days to 14 days. rhTGF-β1 administration decreased the expression of TGF-β1/Smad pathway proteins, except for TGF-β1RI. Nestin and CD29 expression and the number of Sca1(+)CD29(+)CD11b(-)CD45(-) cells were reduced, whereas MMP-14 expression was increased after SB431542 and rhTGF-β1 administration. These results suggest that TGF-β1/Smad signalling and MMP-14 act to recruit MSCs which differentiate to vascular smooth muscle cells and mesenchymal-like cells that participate in arterial repair/remodelling.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
TGF-β1/Smad signaling, MMP-14, and MSC markers in arterial injury: discovery of the molecular basis of restenosis.Int J Clin Exp Pathol. 2014 May 15;7(6):2915-24. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25031710 Free PMC article.
-
Human urine kininogenase attenuates balloon-induced intimal hyperplasia in rabbit carotid artery through transforming growth factor β1/Smad2/3 signaling pathway.J Vasc Surg. 2016 Oct;64(4):1074-83. doi: 10.1016/j.jvs.2015.04.433. Epub 2015 Jun 6. J Vasc Surg. 2016. PMID: 26054589
-
Osthole inhibits intimal hyperplasia by regulating the NF-κB and TGF-β1/Smad2 signalling pathways in the rat carotid artery after balloon injury.Eur J Pharmacol. 2017 Sep 15;811:232-239. doi: 10.1016/j.ejphar.2017.06.025. Epub 2017 Jun 23. Eur J Pharmacol. 2017. PMID: 28648404
-
LMO7 Is a Negative Feedback Regulator of Transforming Growth Factor β Signaling and Fibrosis.Circulation. 2019 Jan 29;139(5):679-693. doi: 10.1161/CIRCULATIONAHA.118.034615. Circulation. 2019. PMID: 30586711 Free PMC article.
-
The role of progenitor cells in the development of intimal hyperplasia.J Vasc Surg. 2009 Feb;49(2):502-10. doi: 10.1016/j.jvs.2008.07.060. Epub 2008 Oct 22. J Vasc Surg. 2009. PMID: 18945574 Free PMC article. Review.
Cited by
-
An Ex Vivo Vessel Injury Model to Study Remodeling.Cell Transplant. 2018 Sep;27(9):1375-1389. doi: 10.1177/0963689718792201. Epub 2018 Aug 10. Cell Transplant. 2018. PMID: 30095004 Free PMC article.
-
Ras homolog family member A/Rho-associated protein kinase 1 signaling modulates lineage commitment of mesenchymal stem cells in asthmatic patients through lymphoid enhancer-binding factor 1.J Allergy Clin Immunol. 2019 Apr;143(4):1560-1574.e6. doi: 10.1016/j.jaci.2018.08.023. Epub 2018 Sep 5. J Allergy Clin Immunol. 2019. PMID: 30194990 Free PMC article.
-
Extracellular vesicles derived from SARS-CoV-2 M-protein-induced triple negative breast cancer cells promoted the ability of tissue stem cells supporting cancer progression.Front Oncol. 2024 Mar 7;14:1346312. doi: 10.3389/fonc.2024.1346312. eCollection 2024. Front Oncol. 2024. PMID: 38515582 Free PMC article.
-
Bone Marrow-Derived Mesenchymal Stem Cells Migrate toward Hormone-Insensitive Prostate Tumor Cells Expressing TGF-β via N-Cadherin.Biomedicines. 2021 Oct 29;9(11):1572. doi: 10.3390/biomedicines9111572. Biomedicines. 2021. PMID: 34829800 Free PMC article.
-
PEGylated Polyethylenimine Derivative-Mediated Local Delivery of the shSmad3 Inhibits Intimal Thickening after Vascular Injury.Biomed Res Int. 2019 Jul 29;2019:8483765. doi: 10.1155/2019/8483765. eCollection 2019. Biomed Res Int. 2019. Retraction in: Biomed Res Int. 2025 Jul 30;2025:9808963. doi: 10.1155/bmri/9808963. PMID: 31467913 Free PMC article. Retracted.
References
-
- Colin D. M., Ties B. & Doris M. F. Global and regional causes of death. Br Med Bul 92, 7–32 (2009). - PubMed
-
- O’Brien E. R., Ma X., Simard T., Pourdjabbar A. & Hibbert B. Pathogenesis of neointimal formation following vascular injury. CardiovascHematolDisord Drug Targets 11, 30–39 (2011). - PubMed
-
- Merzkirch C., Davies N. & Zilla P. Engineering of vascular in-growth matrices: are protein domains an alternative to peptides? Anat Rec 263, 379–387 (2001). - PubMed
-
- Douglas J. S. Jr. Pharmacologic approaches to restenosis prevention. Am J Cardiol 100, 10K–6K (2007). - PubMed
-
- Kipshidze N. et al. Endoluminal reconstruction of the arterial wall with endothelial cell/qlue matrix reduces restenosis in an atherosclerotic rabbit. J Am Coll Cardiol 36, 1396–1403 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

