Tuftsin-driven experimental autoimmune encephalomyelitis recovery requires neuropilin-1
- PMID: 26880314
- PMCID: PMC4833601
- DOI: 10.1002/glia.22972
Tuftsin-driven experimental autoimmune encephalomyelitis recovery requires neuropilin-1
Abstract
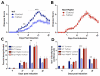
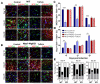
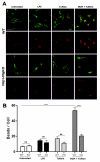
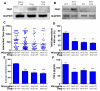
Experimental autoimmune encephalomyelitis (EAE) is an animal model of demyelinating autoimmune disease, such as multiple sclerosis (MS), which is characterized by central nervous system white matter lesions, microglial activation, and peripheral T-cell infiltration secondary to blood-brain barrier disruption. We have previously shown that treatment with tuftsin, a tetrapeptide generated from IgG proteolysis, dramatically improves disease symptoms in EAE. Here, we report that microglial expression of Neuropilin-1 (Nrp1) is required for tuftsin-driven amelioration of EAE symptoms. Nrp1 ablation in microglia blocks microglial signaling and polarization to the anti-inflammatory M2 phenotype, and ablation in either the microglia or immunosuppressive regulatory T cells (Tregs) reduces extended functional contacts between them and Treg activation, implicating a role for microglia in the activation process, and more generally, how immune surveillance is conducted in the CNS. Taken together, our findings delineate the mechanistic action of tuftsin as a candidate therapeutic against immune-mediated demyelinating lesions.
Keywords: EAE; Treg; anti-inflammatory; mice; microglia.
© 2016 Wiley Periodicals, Inc.
Figures
Similar articles
-
Modulation of microglial/macrophage activation by macrophage inhibitory factor (TKP) or tuftsin (TKPR) attenuates the disease course of experimental autoimmune encephalomyelitis.BMC Immunol. 2007 Jul 16;8:10. doi: 10.1186/1471-2172-8-10. BMC Immunol. 2007. PMID: 17634104 Free PMC article.
-
Tuftsin promotes an anti-inflammatory switch and attenuates symptoms in experimental autoimmune encephalomyelitis.PLoS One. 2012;7(4):e34933. doi: 10.1371/journal.pone.0034933. Epub 2012 Apr 17. PLoS One. 2012. PMID: 22529957 Free PMC article.
-
Tuftsin signals through its receptor neuropilin-1 via the transforming growth factor beta pathway.J Neurochem. 2013 Nov;127(3):394-402. doi: 10.1111/jnc.12404. Epub 2013 Sep 18. J Neurochem. 2013. PMID: 24033337 Free PMC article.
-
Macrophages: a double-edged sword in experimental autoimmune encephalomyelitis.Immunol Lett. 2014 Jul;160(1):17-22. doi: 10.1016/j.imlet.2014.03.006. Epub 2014 Mar 31. Immunol Lett. 2014. PMID: 24698730 Free PMC article. Review.
-
The roles of macrophages and microglia in multiple sclerosis and experimental autoimmune encephalomyelitis.J Neuroimmunol. 2018 May 15;318:1-7. doi: 10.1016/j.jneuroim.2018.02.015. Epub 2018 Feb 27. J Neuroimmunol. 2018. PMID: 29606295 Review.
Cited by
-
Visualizing the Brain's Astrocytes with Diverse Chemical Scaffolds.ACS Chem Biol. 2018 Jun 15;13(6):1493-1498. doi: 10.1021/acschembio.8b00391. Epub 2018 May 9. ACS Chem Biol. 2018. PMID: 29733639 Free PMC article.
-
Cre Driver Mice Targeting Macrophages.Methods Mol Biol. 2018;1784:263-275. doi: 10.1007/978-1-4939-7837-3_24. Methods Mol Biol. 2018. PMID: 29761406 Free PMC article.
-
Helminths-based bi-functional molecule, tuftsin-phosphorylcholine (TPC), ameliorates an established murine arthritis.PLoS One. 2018 Aug 8;13(8):e0200615. doi: 10.1371/journal.pone.0200615. eCollection 2018. PLoS One. 2018. PMID: 30089122 Free PMC article.
-
The emerging role of galectins in (re)myelination and its potential for developing new approaches to treat multiple sclerosis.Cell Mol Life Sci. 2020 Apr;77(7):1289-1317. doi: 10.1007/s00018-019-03327-7. Epub 2019 Oct 18. Cell Mol Life Sci. 2020. PMID: 31628495 Free PMC article. Review.
-
Guanabenz modulates microglia and macrophages during demyelination.Sci Rep. 2020 Nov 9;10(1):19333. doi: 10.1038/s41598-020-76383-w. Sci Rep. 2020. PMID: 33168944 Free PMC article.
References
-
- Almolda B, Gonzalez B, Castellano B. Antigen presentation in EAE: role of microglia, macrophages and dendritic cells. Front Biosci. 2011;16:1157–71. - PubMed
-
- Anthonypillai C, Sanderson RN, Gibbs JE, Thomas SA. The distribution of the HIV protease inhibitor, ritonavir, to the brain, cerebrospinal fluid, and choroid plexuses of the guinea pig. J Pharmacol Exp Ther. 2004;308:912–20. - PubMed
-
- Arno B, Grassivaro F, Rossi C, Bergamaschi A, Castiglioni V, Furlan R, Greter M, Favaro R, Comi G, Becher B. Neural progenitor cells orchestrate microglia migration and positioning into the developing cortex. Nat Commun. 2014;5:5611. others. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

