A review of the proposed role of neutrophils in rodent amebic liver abscess models
- PMID: 26880421
- PMCID: PMC4754534
- DOI: 10.1051/parasite/2016006
A review of the proposed role of neutrophils in rodent amebic liver abscess models
Abstract
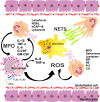
Host invasion by Entamoeba histolytica, the pathogenic agent of amebiasis, can lead to the development of amebic liver abscess (ALA). Due to the difficulty of exploring host and amebic factors involved in the pathogenesis of ALA in humans, most studies have been conducted with animal models (e.g., mice, gerbils, and hamsters). Histopathological findings reveal that the chronic phase of ALA in humans corresponds to lytic or liquefactive necrosis, whereas in rodent models there is granulomatous inflammation. However, the use of animal models has provided important information on molecules and mechanisms of the host/parasite interaction. Hence, the present review discusses the possible role of neutrophils in the effector immune response in ALA in rodents. Properly activated neutrophils are probably successful in eliminating amebas through oxidative and non-oxidative mechanisms, including neutrophil degranulation, the generation of free radicals (O2(-), H2O2, HOCl) and peroxynitrite, the activation of NADPH-oxidase and myeloperoxidase (MPO) enzymes, and the formation of neutrophil extracellular traps (NETs). On the other hand, if amebas are not eliminated in the early stages of infection, they trigger a prolonged and exaggerated inflammatory response that apparently causes ALAs. Genetic differences in animals and humans are likely to be key to a successful host immune response.
L’invasion d’un hôte par Entamoeba histolytica, l’agent pathogène de l’amibiase, peut conduire au développement d’un abcès hépatique amibien (AHA). En raison de la difficulté d’explorer les facteurs dépendant de l’hôte et des amibes impliqués dans la pathogenèse de l’AHA chez les humains, la plupart des études ont été menées sur des modèles animaux (par exemple souris, gerbilles et hamsters). Les résultats histopathologiques montrent que la phase chronique de l’AHA chez l’homme correspond à la nécrose lytique ou liquéfiante, tandis que dans les modèles rongeurs on rencontre une inflammation granulomateuse. Cependant, l’utilisation de modèles animaux a fourni des informations importantes sur les molécules et les mécanismes de l’interaction hôte/parasite. Cette synthèse discute donc le rôle possible des neutrophiles dans la réponse immunitaire effectrice pendant l’AHA chez les rongeurs. Les neutrophiles correctement activés réussissent probablement à éliminer les amibes grâce à des mécanismes oxydatifs et non oxydatifs, y compris la dégranulation des neutrophiles, la génération de radicaux libres (O2−, H2O2, HOCl) et de peroxynitrite, l’activation des enzymes NADPH-oxydase et myéloperoxydase, et la formation de pièges extracellulaires des neutrophiles. D’autre part, si les amibes ne sont pas éliminées au cours des premiers stades de l’infection, elles déclenchent une réponse inflammatoire prolongée et excessive qui provoque apparemment l’AHA. Les différences génétiques chez les animaux et les humains sont probablement la clé d’une réponse immunitaire réussie de l’hôte.
© R. Campos-Rodríguez et al., published by EDP Sciences, 2016.
Figures

Similar articles
-
Neutrophil extracellular traps and MPO in models of susceptibility and resistance against Entamoeba histolytica.Parasite Immunol. 2020 Jun;42(6):e12714. doi: 10.1111/pim.12714. Epub 2020 Apr 7. Parasite Immunol. 2020. PMID: 32187688
-
Different behavior of myeloperoxidase in two rodent amoebic liver abscess models.PLoS One. 2017 Aug 10;12(8):e0182480. doi: 10.1371/journal.pone.0182480. eCollection 2017. PLoS One. 2017. PMID: 28796788 Free PMC article.
-
Morphological characterization of experimental amebic liver lesions in gerbils.Arch Med Res. 1992;23(2):203-7. Arch Med Res. 1992. PMID: 1340295
-
Peroxynitrite and peroxiredoxin in the pathogenesis of experimental amebic liver abscess.Biomed Res Int. 2014;2014:324230. doi: 10.1155/2014/324230. Epub 2014 Apr 15. Biomed Res Int. 2014. PMID: 24822193 Free PMC article. Review.
-
Pathogenesis of acute experimental liver amebiasis.Arch Med Res. 2006 Feb;37(2):203-9. doi: 10.1016/j.arcmed.2005.10.007. Arch Med Res. 2006. PMID: 16380320 Review.
Cited by
-
Apocynin, an NADPH Oxidase Enzyme Inhibitor, Prevents Amebic Liver Abscess in Hamster.Biomedicines. 2023 Aug 21;11(8):2322. doi: 10.3390/biomedicines11082322. Biomedicines. 2023. PMID: 37626818 Free PMC article.
-
Autophagy Activated by Peroxiredoxin of Entamoeba histolytica.Cells. 2020 Nov 12;9(11):2462. doi: 10.3390/cells9112462. Cells. 2020. PMID: 33198056 Free PMC article.
-
New Insights on NETosis Induced by Entamoeba histolytica: Dependence on ROS from Amoebas and Extracellular MPO Activity.Antioxidants (Basel). 2021 Jun 18;10(6):974. doi: 10.3390/antiox10060974. Antioxidants (Basel). 2021. PMID: 34206992 Free PMC article.
-
Host/genetic factors associated with COVID-19 call for precision medicine.Precis Clin Med. 2020 Jul 21;3(3):228-234. doi: 10.1093/pcmedi/pbaa026. eCollection 2020 Sep. Precis Clin Med. 2020. PMID: 35960669 Free PMC article.
-
Curcumin Provides Hepatoprotection against Amoebic Liver Abscess Induced by Entamoeba histolytica in Hamster: Involvement of Nrf2/HO-1 and NF-κB/IL-1β Signaling Pathways.J Immunol Res. 2019 Apr 15;2019:7431652. doi: 10.1155/2019/7431652. eCollection 2019. J Immunol Res. 2019. PMID: 31275999 Free PMC article.
References
-
- Aguirre-Garcia J. 1970. Histopathological peculiarities of the amebic lesion. Archivos de Investigacion Medica (Mex), 1(Suppl), 147–156. - PubMed
-
- Akbar MA, Chatterjee NS, Sen P, Debnath A, Pal A, Bera T, Das P. 2004. Genes induced by a high-oxygen environment in Entamoeba histolytica. Molecular and Biochemical Parasitology, 133(2), 187–196. - PubMed
-
- Al-Mofleh IA, Al-Tuwaijri AS, Mahmoud AA, Alam M. 1989. Entamoeba histolytica depresses chemiluminescence in stimulated human polymorphonuclear leukocytes. International Journal of Immunopharmacology, 11(5), 529–536. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
