Structural insights into the mechanism of activation of the TRPV1 channel by a membrane-bound tarantula toxin
- PMID: 26880553
- PMCID: PMC4764579
- DOI: 10.7554/eLife.11273
Structural insights into the mechanism of activation of the TRPV1 channel by a membrane-bound tarantula toxin
Abstract
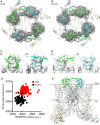
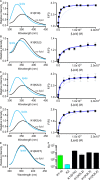
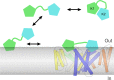
Venom toxins are invaluable tools for exploring the structure and mechanisms of ion channels. Here, we solve the structure of double-knot toxin (DkTx), a tarantula toxin that activates the heat-activated TRPV1 channel. We also provide improved structures of TRPV1 with and without the toxin bound, and investigate the interactions of DkTx with the channel and membranes. We find that DkTx binds to the outer edge of the external pore of TRPV1 in a counterclockwise configuration, using a limited protein-protein interface and inserting hydrophobic residues into the bilayer. We also show that DkTx partitions naturally into membranes, with the two lobes exhibiting opposing energetics for membrane partitioning and channel activation. Finally, we find that the toxin disrupts a cluster of hydrophobic residues behind the selectivity filter that are critical for channel activation. Collectively, our findings reveal a novel mode of toxin-channel recognition that has important implications for the mechanism of thermosensation.
Keywords: biophysics; capsaicin; membrane channel; membrane structure; protein-protein interactions in membranes; rat; structural biology; thermosensing.
Conflict of interest statement
KJS: Reviewing editor,
The other authors declare that no competing interests exist.
Figures













Similar articles
-
Dissecting the contributions of membrane affinity and bivalency of the spider venom protein DkTx to its sustained mode of TRPV1 activation.J Biol Chem. 2023 Jul;299(7):104903. doi: 10.1016/j.jbc.2023.104903. Epub 2023 Jun 10. J Biol Chem. 2023. PMID: 37302551 Free PMC article.
-
TRPV1 pore turret dictates distinct DkTx and capsaicin gating.Proc Natl Acad Sci U S A. 2018 Dec 11;115(50):E11837-E11846. doi: 10.1073/pnas.1809662115. Epub 2018 Nov 21. Proc Natl Acad Sci U S A. 2018. PMID: 30463948 Free PMC article.
-
A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain.Cell. 2010 May 28;141(5):834-45. doi: 10.1016/j.cell.2010.03.052. Cell. 2010. PMID: 20510930 Free PMC article.
-
Animal Toxins Providing Insights into TRPV1 Activation Mechanism.Toxins (Basel). 2017 Oct 16;9(10):326. doi: 10.3390/toxins9100326. Toxins (Basel). 2017. PMID: 29035314 Free PMC article. Review.
-
Ion-channel modulators: more diversity than previously thought.Chembiochem. 2011 Aug 16;12(12):1808-12. doi: 10.1002/cbic.201100236. Epub 2011 Jul 1. Chembiochem. 2011. PMID: 21726033 Review.
Cited by
-
Structural Basis of the Bivalency of the TRPV1 Agonist DkTx.Angew Chem Int Ed Engl. 2024 Jan 15;63(3):e202314621. doi: 10.1002/anie.202314621. Epub 2023 Dec 12. Angew Chem Int Ed Engl. 2024. PMID: 37953402 Free PMC article.
-
The envenomation of general physiology throughout the last century.J Gen Physiol. 2017 Nov 6;149(11):975-983. doi: 10.1085/jgp.201711856. Epub 2017 Oct 11. J Gen Physiol. 2017. PMID: 29021149 Free PMC article. Review.
-
TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action.Nature. 2016 Jun 16;534(7607):347-51. doi: 10.1038/nature17964. Epub 2016 May 18. Nature. 2016. PMID: 27281200 Free PMC article.
-
Capsaicin in Metabolic Syndrome.Nutrients. 2018 May 17;10(5):630. doi: 10.3390/nu10050630. Nutrients. 2018. PMID: 29772784 Free PMC article. Review.
-
Heat-dependent opening of TRPV1 in the presence of capsaicin.Nat Struct Mol Biol. 2021 Jul;28(7):554-563. doi: 10.1038/s41594-021-00616-3. Epub 2021 Jul 8. Nat Struct Mol Biol. 2021. PMID: 34239123 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

