Selective Targeting of Heme Protein in Cytochrome P450 and Nitric Oxide Synthase by Diphenyleneiodonium
- PMID: 26880746
- PMCID: PMC4914801
- DOI: 10.1093/toxsci/kfw031
Selective Targeting of Heme Protein in Cytochrome P450 and Nitric Oxide Synthase by Diphenyleneiodonium
Abstract
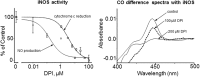
Cytochrome P450 (CYP) enzymes mediate mixed-function oxidation reactions important in drug metabolism. The aromatic heterocyclic cation, diphenyleneiodonium (DPI), binds flavin in cytochrome P450 reductase and inhibits CYP-mediated activity. DPI also inhibits CYP by directly interacting with heme. Herein, we report that DPI effectively inhibits a number of CYP-related monooxygenase reactions including NADPH oxidase, a microsomal enzyme activity that generates hydrogen peroxide in the absence of metabolizing substrates. Inhibition of monooxygenase by DPI was time and concentration dependent with IC50's ranging from 0.06 to 1.9 μM. Higher (4.6-23.9 μM), but not lower (0.06-1.9 μM), concentrations of DPI inhibited electron flow via cytochrome P450 reductase, as measured by its ability to reduce cytochrome c and mediate quinone redox cycling. Similar results were observed with inducible nitric oxide synthase (iNOS), an enzyme containing a C-terminal reductase domain homologous to cytochrome P450 reductase that mediates reduction of cytochrome c, and an N-terminal heme-thiolate oxygenase domain mediating nitric oxide production. Significantly greater concentrations of DPI were required to inhibit cytochrome c reduction by iNOS (IC50 = 3.5 µM) than nitric oxide production (IC50 = 0.16 µM). Difference spectra of liver microsomes, recombinant CYPs, and iNOS demonstrated that DPI altered heme-carbon monoxide interactions. In the presence of NADPH, DPI treatment of microsomes and iNOS yielded a type II spectral shift. These data indicate that DPI interacts with both flavin and heme in CYPs and iNOS. Increased sensitivity for inhibition of CYP-mediated metabolism and nitric oxide production by iNOS indicates that DPI targets heme moieties within the enzymes.
Keywords: cytochrome P450; flavoenzymes; heme; nitric oxide synthase; reactive oxygen species.
© The Author 2016. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures



Similar articles
-
Distinct effects of form selective cytochrome P450 inhibitors on cytochrome P450-mediated monooxygenase and hydrogen peroxide generating NADPH oxidase.Toxicol Appl Pharmacol. 2022 Nov 15;455:116258. doi: 10.1016/j.taap.2022.116258. Epub 2022 Sep 26. Toxicol Appl Pharmacol. 2022. PMID: 36174671
-
Hepatic cytochrome P450 is directly inactivated by nitric oxide, not by inflammatory cytokines, in the early phase of endotoxemia.J Hepatol. 1999 Jun;30(6):1035-44. doi: 10.1016/s0168-8278(99)80257-8. J Hepatol. 1999. PMID: 10406181
-
Inhibition of NADPH-cytochrome P450 reductase and glyceryl trinitrate biotransformation by diphenyleneiodonium sulfate.Biochem Pharmacol. 1998 Oct 1;56(7):881-93. doi: 10.1016/s0006-2952(98)00216-0. Biochem Pharmacol. 1998. PMID: 9774150
-
NOx and R-NOx: effects on drug metabolism.Curr Drug Metab. 2004 Dec;5(6):535-42. doi: 10.2174/1389200043335388. Curr Drug Metab. 2004. PMID: 15578946 Review.
-
Regulation of cytochrome P450 enzyme activity and expression by nitric oxide in the context of inflammatory disease.Drug Metab Rev. 2020 Nov;52(4):455-471. doi: 10.1080/03602532.2020.1817061. Epub 2020 Sep 8. Drug Metab Rev. 2020. PMID: 32898444 Free PMC article. Review.
Cited by
-
Quinone and nitrofurantoin redox cycling by recombinant cytochrome b5 reductase.Toxicol Appl Pharmacol. 2018 Nov 15;359:102-107. doi: 10.1016/j.taap.2018.09.011. Epub 2018 Sep 15. Toxicol Appl Pharmacol. 2018. PMID: 30222979 Free PMC article.
-
Monooxygenase System and NO Metabolism in Liver Microsomes of Rats with Toxic Hepatitis and the Effect of Sesquiterpene Lactones.Bull Exp Biol Med. 2021 Dec;172(2):133-136. doi: 10.1007/s10517-021-05349-3. Epub 2021 Dec 2. Bull Exp Biol Med. 2021. PMID: 34853965
-
Stable Chinese Hamster Ovary Suspension Cell Lines Harboring Recombinant Human Cytochrome P450 Oxidoreductase and Human Cytochrome P450 Monooxygenases as Platform for In Vitro Biotransformation Studies.Cells. 2023 Aug 24;12(17):2140. doi: 10.3390/cells12172140. Cells. 2023. PMID: 37681872 Free PMC article.
-
Genetic Polymorphisms Complicate COVID-19 Therapy: Pivotal Role of HO-1 in Cytokine Storm.Antioxidants (Basel). 2020 Jul 18;9(7):636. doi: 10.3390/antiox9070636. Antioxidants (Basel). 2020. PMID: 32708430 Free PMC article. Review.
-
Mitochondria-targeted antioxidant SkQ1 inhibits leukotriene synthesis in human neutrophils.Front Pharmacol. 2022 Nov 24;13:1023517. doi: 10.3389/fphar.2022.1023517. eCollection 2022. Front Pharmacol. 2022. PMID: 36506526 Free PMC article.
References
-
- Adak S., Ghosh S., Abu-Soud H. M., Stuehr D. J. (1999). Role of reductase domain cluster 1 acidic residues in neuronal nitric-oxide synthase. Characterization of the FMN-free enzyme. J Biol Chem 274, 22313–22320. - PubMed
-
- Aggarwal V. K., Olofsson B. (2005). Enantioselective alpha-arylation of cyclohexanones with diaryl iodonium salts: application to the synthesis of (-)-epibatidine. Angew Chem Int Ed Engl 44, 5516–5519. - PubMed
-
- Banks D. F. (1966). Organic polyvalent iodine compounds. Chem. Rev. 66, 243–266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

