Infectious diseases of marine molluscs and host responses as revealed by genomic tools
- PMID: 26880838
- PMCID: PMC4760136
- DOI: 10.1098/rstb.2015.0206
Infectious diseases of marine molluscs and host responses as revealed by genomic tools
Abstract
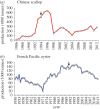
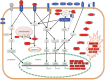
More and more infectious diseases affect marine molluscs. Some diseases have impacted commercial species including MSX and Dermo of the eastern oyster, QPX of hard clams, withering syndrome of abalone and ostreid herpesvirus 1 (OsHV-1) infections of many molluscs. Although the exact transmission mechanisms are not well understood, human activities and associated environmental changes often correlate with increased disease prevalence. For instance, hatcheries and large-scale aquaculture create high host densities, which, along with increasing ocean temperature, might have contributed to OsHV-1 epizootics in scallops and oysters. A key to understanding linkages between the environment and disease is to understand how the environment affects the host immune system. Although we might be tempted to downplay the role of immunity in invertebrates, recent advances in genomics have provided insights into host and parasite genomes and revealed surprisingly sophisticated innate immune systems in molluscs. All major innate immune pathways are found in molluscs with many immune receptors, regulators and effectors expanded. The expanded gene families provide great diversity and complexity in innate immune response, which may be key to mollusc's defence against diverse pathogens in the absence of adaptive immunity. Further advances in host and parasite genomics should improve our understanding of genetic variation in parasite virulence and host disease resistance.
Keywords: aquaculture; genomics; immune response; marine diseases; mollusc; parasites.
© 2016 The Author(s).
Figures
References
-
- Guo X, Ford S, Zhang F. 1999. Molluscan aquaculture in China. J. Shellfish Res. 18, 19–31.
-
- Elston RA. 1990. Mollusc diseases: guide for the shellfish farmer. Seattle, WA: Washington Sea Grant.
-
- Van Valen L. 1973. A new evolutionary law. Evol. Theory 1, 1–30.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
