Chalcone Scaffold in Anticancer Armamentarium: A Molecular Insight
- PMID: 26880913
- PMCID: PMC4735904
- DOI: 10.1155/2016/7651047
Chalcone Scaffold in Anticancer Armamentarium: A Molecular Insight
Abstract
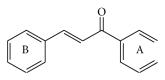
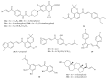
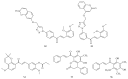
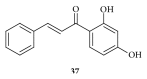
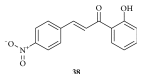
Cancer is an inevitable matter of concern in the medicinal chemistry era. Chalcone is the well exploited scaffold in the anticancer domain. The molecular mechanism of chalcone at cellular level was explored in past decades. This mini review provides the most recent updates on anticancer potential of chalcones.
Figures
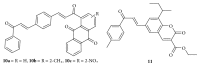
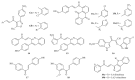
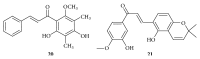
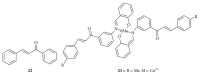
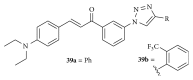
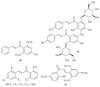
Similar articles
-
Heterocyclic chalcone analogues as potential anticancer agents.Anticancer Agents Med Chem. 2013 Mar;13(3):422-32. Anticancer Agents Med Chem. 2013. PMID: 22721390 Review.
-
Recent developments in biological activities of chalcones: a mini review.Eur J Med Chem. 2014 Oct 6;85:758-77. doi: 10.1016/j.ejmech.2014.08.033. Epub 2014 Aug 12. Eur J Med Chem. 2014. PMID: 25137491 Review.
-
Advances in chalcones with anticancer activities.Recent Pat Anticancer Drug Discov. 2015;10(1):97-115. doi: 10.2174/1574892809666140819153902. Recent Pat Anticancer Drug Discov. 2015. PMID: 25138130 Review.
-
Targeting triple negative breast cancer heterogeneity with chalcones: a molecular insight.J Drug Target. 2019 Sep;27(8):830-838. doi: 10.1080/1061186X.2018.1561889. Epub 2019 Feb 11. J Drug Target. 2019. PMID: 30582377 Review.
-
Exploring pharmacological significance of chalcone scaffold: a review.Curr Med Chem. 2012;19(2):209-25. doi: 10.2174/092986712803414132. Curr Med Chem. 2012. PMID: 22320299 Review.
Cited by
-
Multienzymatic biotransformation of flavokawain B by entomopathogenic filamentous fungi: structural modifications and pharmacological predictions.Microb Cell Fact. 2024 Feb 24;23(1):65. doi: 10.1186/s12934-024-02338-9. Microb Cell Fact. 2024. PMID: 38402203 Free PMC article.
-
The effect of novel nitrogen-based chalcone analogs on colorectal cancer cells: Insight into the molecular pathways.Heliyon. 2024 Feb 27;10(5):e27002. doi: 10.1016/j.heliyon.2024.e27002. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38463818 Free PMC article.
-
Chalcone: A Privileged Structure in Medicinal Chemistry.Chem Rev. 2017 Jun 28;117(12):7762-7810. doi: 10.1021/acs.chemrev.7b00020. Epub 2017 May 10. Chem Rev. 2017. PMID: 28488435 Free PMC article. Review.
-
Molecular targets and anticancer activity of quinoline-chalcone hybrids: literature review.RSC Adv. 2020 Aug 21;10(52):31139-31155. doi: 10.1039/d0ra05594h. eCollection 2020 Aug 21. RSC Adv. 2020. PMID: 35520674 Free PMC article. Review.
-
Excavating medicinal virtues of chalcones to illuminate a new scope in cancer chemotherapy.RSC Adv. 2025 Apr 14;15(15):11617-11638. doi: 10.1039/d5ra01280e. eCollection 2025 Apr 9. RSC Adv. 2025. PMID: 40230627 Free PMC article. Review.
References
-
- American Cancer Society. Global Cancer Facts & Figures. 2nd. Atlanta, Ga, USA: American Cancer Society; 2011.
-
- Alison M. R. Cancer. Encyclopedia of life sciences, Nature Publishing Group, 2011, http://www.els.net.
-
- Dhar D. N. The Chemistry of Chalcones and Related Compounds. New York, NY, USA: John Wiley & Sons; 1981.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

