Enantioseparation of Citalopram by RP-HPLC, Using Sulfobutyl Ether-β-Cyclodextrin as a Chiral Mobile Phase Additive
- PMID: 26880921
- PMCID: PMC4736382
- DOI: 10.1155/2016/1231386
Enantioseparation of Citalopram by RP-HPLC, Using Sulfobutyl Ether-β-Cyclodextrin as a Chiral Mobile Phase Additive
Abstract
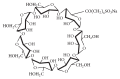
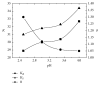
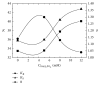
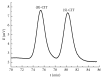
Enantiomeric separation of citalopram (CIT) was developed using a reversed phase HPLC (RP-HPLC) with sulfobutylether-β-cyclodextrin (SBE-β-CD) as a chiral mobile phase additive. The effects of the pH value of aqueous buffer, concentration of chiral additive, composition of mobile phase, and column temperature on the enantioseparation of CIT were investigated on the Hedera ODS-2 C18 column (250 mm × 4.6 mm × 5.0 um). A satisfactory resolution was achieved at 25°C using a mobile phase consisting of a mixture of aqueous buffer (pH of 2.5, 5 mM sodium dihydrogen phosphate, and 12 mM SBE-β-CD), methanol, and acetonitrile with a volumetric ratio of 21 : 3 : 1 and flow rate of 1.0 mL/min. This analytical method was evaluated by examining the precision (lower than 3.0%), linearity (regression coefficients close to 1), limit of detection (0.070 µg/mL for (R)-CIT and 0.076 µg/mL for (S)-CIT), and limit of quantitation (0.235 µg/mL for (R)-CIT and 0.254 µg/mL for (S)-CIT).
Figures
Similar articles
-
[High performance liquid chromatographic enantioseparation of atrolactic acids using chiral mobile phase additive].Se Pu. 2014 Jun;32(6):612-5. doi: 10.3724/sp.j.1123.2014.02023. Se Pu. 2014. PMID: 25269259 Chinese.
-
[Separation of ketoconazole enantiomers using high performance liquid chromatography with chiral mobile phase additives].Se Pu. 2009 Mar;27(2):240-3. Se Pu. 2009. PMID: 19626858 Chinese.
-
Enantioseparation of mandelic acid derivatives by high performance liquid chromatography with substituted β-cyclodextrin as chiral mobile phase additive and evaluation of inclusion complex formation.J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Jul 1;962:44-51. doi: 10.1016/j.jchromb.2014.05.026. Epub 2014 May 22. J Chromatogr B Analyt Technol Biomed Life Sci. 2014. PMID: 24893270 Free PMC article.
-
Determination of the enantiomeric impurity in S-(-)pantoprazole using high performance liquid chromatography with sulfobutylether-beta-cyclodextrin as chiral additive.J Sep Sci. 2008 Feb;31(2):288-93. doi: 10.1002/jssc.200700369. J Sep Sci. 2008. PMID: 18196525
-
[Separation of racemic flavonoids by high performance liquid chromatography using chiral mobile phase additive].Se Pu. 2013 May;31(5):447-50. doi: 10.3724/sp.j.1123.2012.12054. Se Pu. 2013. PMID: 24010344 Chinese.
Cited by
-
Emerging Developments in Separation Techniques and Analysis of Chiral Pharmaceuticals.Molecules. 2023 Aug 22;28(17):6175. doi: 10.3390/molecules28176175. Molecules. 2023. PMID: 37687004 Free PMC article. Review.
-
Quality-by-Design Is a Tool for Quality Assurance in the Assessment of Enantioseparation of a Model Active Pharmaceutical Ingredient.Pharmaceuticals (Basel). 2020 Nov 4;13(11):364. doi: 10.3390/ph13110364. Pharmaceuticals (Basel). 2020. PMID: 33158197 Free PMC article.
-
Enantioselectivity in Drug Pharmacokinetics and Toxicity: Pharmacological Relevance and Analytical Methods.Molecules. 2021 May 23;26(11):3113. doi: 10.3390/molecules26113113. Molecules. 2021. PMID: 34070985 Free PMC article. Review.
-
Interpol review of controlled substances 2016-2019.Forensic Sci Int Synerg. 2020 May 24;2:608-669. doi: 10.1016/j.fsisyn.2020.01.019. eCollection 2020. Forensic Sci Int Synerg. 2020. PMID: 33385148 Free PMC article. Review.
-
Establishment and Validation of a Transdermal Drug Delivery System for the Anti-Depressant Drug Citalopram Hydrobromide.Molecules. 2024 Feb 7;29(4):767. doi: 10.3390/molecules29040767. Molecules. 2024. PMID: 38398519 Free PMC article.
References
-
- Petersen H. Method for the preparation of citalopram. WO 9819513A2, 1998.
LinkOut - more resources
Full Text Sources
Other Literature Sources

