Cancer Stem Cell Signaling during Repopulation in Head and Neck Cancer
- PMID: 26880935
- PMCID: PMC4736761
- DOI: 10.1155/2016/1894782
Cancer Stem Cell Signaling during Repopulation in Head and Neck Cancer
Abstract
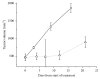
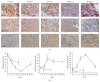
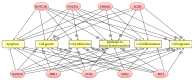
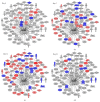
The aim of the study was to investigate cancer stem signaling during the repopulation response of a head and neck squamous cell cancer (HNSCC) xenograft after radiation treatment. Xenografts were generated from low passage HNSCC cells and were treated with either sham radiation or 15 Gy in one fraction. At different time points, days 0, 3, and 10 for controls and days 4, 7, 12, and 21, after irradiation, 3 tumors per group were harvested for global gene expression, pathway analysis, and immunohistochemical evaluation. 316 genes were identified that were associated with a series of stem cell-related genes and were differentially expressed (p ≤ 0.01 and 1.5-fold) at a minimum of one time point in UT-SCC-14 xenografts after radiation. The largest network of genes that showed significant changes after irradiation was associated with CD44, NOTCH1, and MET. c-MET and ALDH1A3 staining correlated with the changes in gene expression. A clear pattern emerged that was consistent with the growth inhibition data in that genes associated with stem cell pathways were most active at day 7 and day 12 after irradiation. The MET/CD44 axis seemed to be an important component of the repopulation response.
Figures
References
-
- Bryne M., Boysen M., Alfsen C. G., et al. The invasive front of carcinomas. The most important area for tumour prognosis? Anticancer Research. 1998;18(6):4757–4764. - PubMed
-
- Tabor M. P., Brakenhoff R. H., Van Houten V. M. M., et al. Persistence of genetically altered fields in head and neck cancer patients: biological and clinical implications. Clinical Cancer Research. 2001;7(6):1523–1532. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

