Comparative Analysis of Human Mesenchymal Stem Cells from Umbilical Cord, Dental Pulp, and Menstrual Blood as Sources for Cell Therapy
- PMID: 26880954
- PMCID: PMC4736971
- DOI: 10.1155/2016/3516574
Comparative Analysis of Human Mesenchymal Stem Cells from Umbilical Cord, Dental Pulp, and Menstrual Blood as Sources for Cell Therapy
Abstract
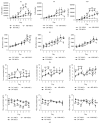
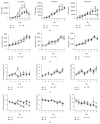
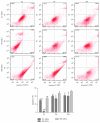
Although mesenchymal stem cells (MSCs) based therapy has been considered as a promising tool for tissue repair and regeneration, the optimal cell source remains unknown. Umbilical cord (UC), dental pulp (DP), and menstrual blood (MB) are easily accessible sources, which make them attractive candidates for MSCs. The goal of this study was to compare the biological characteristics, including morphology, proliferation, antiapoptosis, multilineage differentiation capacity, and immunophenotype of UC-, DP-, and MB-MSCs in order to provide a theoretical basis for clinical selection and application of these cells. As a result, all UC-, DP-, and MB-MSCs have self-renewal capacity and multipotentiality. However, the UC-MSCs seemed to have higher cell proliferation ability, while DP-MSCs may have significant advantages for osteogenic differentiation, lower cell apoptosis, and senescence. These differences may be associated with the different expression level of cytokines, including vascular endothelial growth factor, fibroblast growth factor, keratinocyte growth factor, and hepatocyte growth factor in each of the MSCs. Comprehensively, our results suggest DP-MSCs may be a desired source for clinical applications of cell therapy.
Figures




References
-
- Li Y., Charif N., Mainard D., Bensoussan D., Stoltz J.-F., de Isla N. Donor's age dependent proliferation decrease of human bone marrow mesenchymal stem cells is linked to diminished clonogenicity. Bio-Medical Materials and Engineering. 2014;24(1, supplement):47–52. doi: 10.3233/BME-140973. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

