Improved Protective Effect of Umbilical Cord Stem Cell Transplantation on Cisplatin-Induced Kidney Injury in Mice Pretreated with Antithymocyte Globulin
- PMID: 26880955
- PMCID: PMC4736416
- DOI: 10.1155/2016/3585362
Improved Protective Effect of Umbilical Cord Stem Cell Transplantation on Cisplatin-Induced Kidney Injury in Mice Pretreated with Antithymocyte Globulin
Abstract
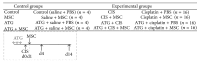
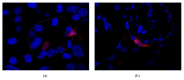
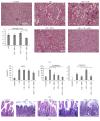
Mesenchymal stem cells (MSCs) are recognised as a promising tool to improve renal recovery in experimental models of cisplatin-induced acute kidney injury. However, these preclinical studies were performed on severely immunodeficient animals. Here, we investigated whether human umbilical cord derived MSC treatment could equally ameliorate acute kidney injury induced by cisplatin and prolong survival in mice with a normal immune system and those with a suppressed immune system by polyclonal antithymocyte globulin (ATG). We demonstrated that ATG pretreatment, when followed by MSC transplantation, significantly improved injured renal function parameters, as evidenced by decreased blood urea nitrogen and serum creatinine concentration, as well as improved renal morphology. This tissue restoration was also supported by increased survival of mice. The beneficial effects of ATG were associated with reduced level of inflammatory protein serum amyloid A3 and induced antioxidative expression of superoxide dismutase-1 (SOD-1), glutathione peroxidase (GPx), and hem oxygenase-1 (HO-1). Infused MSCs became localised predominantly in peritubular areas and acted to reduce renal cell death. In conclusion, these results show that ATG diminished in situ inflammation and oxidative stress associated with cisplatin-induced acute kidney injury, the effects that may provide more favourable microenvironment for MSC action, with consequential synergistic improvements in renal injury and animal survival as compared to MSC treatment alone.
Figures



Similar articles
-
Comparative study of allogenic and xenogeneic mesenchymal stem cells on cisplatin-induced acute kidney injury in Sprague-Dawley rats.Stem Cell Res Ther. 2016 Sep 1;7(1):126. doi: 10.1186/s13287-016-0386-0. Stem Cell Res Ther. 2016. PMID: 27585525 Free PMC article.
-
Mesenchymal Stem Cells Modified with Heme Oxygenase-1 Have Enhanced Paracrine Function and Attenuate Lipopolysaccharide-Induced Inflammatory and Oxidative Damage in Pulmonary Microvascular Endothelial Cells.Cell Physiol Biochem. 2018;49(1):101-122. doi: 10.1159/000492847. Epub 2018 Aug 28. Cell Physiol Biochem. 2018. PMID: 30153667
-
Efficiency of endovenous versus arterial administration of mesenchymal stem cells for ischemia-reperfusion-induced renal dysfunction in rats.Transplant Proc. 2013 Mar;45(2):503-10. doi: 10.1016/j.transproceed.2012.07.162. Transplant Proc. 2013. PMID: 23498785
-
Human umbilical cord mesenchymal stem cells attenuate cisplatin-induced acute and chronic renal injury.Exp Biol Med (Maywood). 2013 Aug 1;238(8):960-70. doi: 10.1177/1535370213497176. Exp Biol Med (Maywood). 2013. PMID: 23970411
-
Early, but not late, treatment with human umbilical cord blood-derived mesenchymal stem cells attenuates cisplatin nephrotoxicity through immunomodulation.Am J Physiol Renal Physiol. 2017 Oct 1;313(4):F984-F996. doi: 10.1152/ajprenal.00097.2016. Epub 2017 Mar 29. Am J Physiol Renal Physiol. 2017. PMID: 28356286
Cited by
-
Recent Advances in Models, Mechanisms, Biomarkers, and Interventions in Cisplatin-Induced Acute Kidney Injury.Int J Mol Sci. 2019 Jun 20;20(12):3011. doi: 10.3390/ijms20123011. Int J Mol Sci. 2019. PMID: 31226747 Free PMC article. Review.
-
Cobalt Ferrite Magnetic Nanoparticles for Tracing Mesenchymal Stem Cells in Tissue: A Preliminary Study.Int J Mol Sci. 2022 Aug 5;23(15):8738. doi: 10.3390/ijms23158738. Int J Mol Sci. 2022. PMID: 35955869 Free PMC article.
-
Modulation of Renal Parenchyma in Response to Allogeneic Adipose-Derived Mesenchymal Stem Cells Transplantation in Acute Kidney Injury.Int J Stem Cells. 2019 Mar 30;12(1):125-138. doi: 10.15283/ijsc18091. Int J Stem Cells. 2019. PMID: 30836723 Free PMC article.
-
(Mesenchymal) Stem Cell-Based Therapy in Cisplatin-Induced Acute Kidney Injury Animal Model: Risk of Immunogenicity and Tumorigenicity.Stem Cells Int. 2017;2017:7304643. doi: 10.1155/2017/7304643. Epub 2017 Dec 12. Stem Cells Int. 2017. PMID: 29379525 Free PMC article. Review.
-
Stem/Stromal Cells for Treatment of Kidney Injuries With Focus on Preclinical Models.Front Med (Lausanne). 2018 Jun 15;5:179. doi: 10.3389/fmed.2018.00179. eCollection 2018. Front Med (Lausanne). 2018. PMID: 29963554 Free PMC article. Review.
References
-
- Eliopoulos N., Zhao J., Bouchentouf M., et al. Human marrow-derived mesenchymal stromal cells decrease cisplatin renotoxicity in vitro and in vivo and enhance survival of mice post-intraperitoneal injection. American Journal of Physiology—Renal Physiology. 2010;299(6):F1288–F1298. doi: 10.1152/ajprenal.00671.2009. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

