Monitoring the Bystander Killing Effect of Human Multipotent Stem Cells for Treatment of Malignant Brain Tumors
- PMID: 26880961
- PMCID: PMC4736564
- DOI: 10.1155/2016/4095072
Monitoring the Bystander Killing Effect of Human Multipotent Stem Cells for Treatment of Malignant Brain Tumors
Abstract
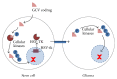
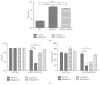
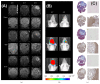
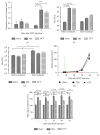
Tumor infiltrating stem cells have been suggested as a vehicle for the delivery of a suicide gene towards otherwise difficult to treat tumors like glioma. We have used herpes simplex virus thymidine kinase expressing human multipotent adult progenitor cells in two brain tumor models (hU87 and Hs683) in immune-compromised mice. In order to determine the best time point for the administration of the codrug ganciclovir, the stem cell distribution and viability were monitored in vivo using bioluminescence (BLI) and magnetic resonance imaging (MRI). Treatment was assessed by in vivo BLI and MRI of the tumors. We were able to show that suicide gene therapy using HSV-tk expressing stem cells can be followed in vivo by MRI and BLI. This has the advantage that (1) outliers can be detected earlier, (2) GCV treatment can be initiated based on stem cell distribution rather than on empirical time points, and (3) a more thorough follow-up can be provided prior to and after treatment of these animals. In contrast to rodent stem cell and tumor models, treatment success was limited in our model using human cell lines. This was most likely due to the lack of immune components in the immune-compromised rodents.
Figures





Similar articles
-
Bystander killing effect of tymidine kinase gene-transduced adult bone marrow stromal cells with ganciclovir on malignant glioma cells.Neurol Med Chir (Tokyo). 2010;50(7):545-53. doi: 10.2176/nmc.50.545. Neurol Med Chir (Tokyo). 2010. PMID: 20671379
-
Suicide Gene Therapy Against Malignant Gliomas by the Local Delivery of Genetically Engineered Umbilical Cord Mesenchymal Stem Cells as Cellular Vehicles.Curr Gene Ther. 2019;19(5):330-341. doi: 10.2174/1566523219666191028103703. Curr Gene Ther. 2019. PMID: 31657679
-
Valproic acid enhances anti-tumor effect of mesenchymal stem cell mediated HSV-TK gene therapy in intracranial glioma.Biochem Biophys Res Commun. 2012 May 11;421(3):585-90. doi: 10.1016/j.bbrc.2012.04.050. Epub 2012 Apr 14. Biochem Biophys Res Commun. 2012. PMID: 22525671
-
Purified herpes simplex virus thymidine kinase retroviral particles: III. Characterization of bystander killing mechanisms in transfected tumor cells.Cancer Gene Ther. 2002 Jan;9(1):87-95. doi: 10.1038/sj.cgt.7700401. Cancer Gene Ther. 2002. PMID: 11916247
-
Suicide gene therapy with Herpes simplex virus thymidine kinase and ganciclovir is enhanced with connexins to improve gap junctions and bystander effects.Histol Histopathol. 2003 Apr;18(2):495-507. doi: 10.14670/HH-18.495. Histol Histopathol. 2003. PMID: 12647801 Review.
Cited by
-
Engineered Mesenchymal Stem Cells as an Anti-Cancer Trojan Horse.Stem Cells Dev. 2016 Oct;25(20):1513-1531. doi: 10.1089/scd.2016.0120. Epub 2016 Sep 7. Stem Cells Dev. 2016. PMID: 27460260 Free PMC article.
-
Molecular Imaging: A Useful Tool for the Development of Natural Killer Cell-Based Immunotherapies.Front Immunol. 2017 Sep 12;8:1090. doi: 10.3389/fimmu.2017.01090. eCollection 2017. Front Immunol. 2017. PMID: 28955332 Free PMC article. Review.
-
Influence of gap junction intercellular communication composed of connexin 43 on the antineoplastic effect of adriamycin in breast cancer cells.Oncol Lett. 2017 Feb;13(2):857-866. doi: 10.3892/ol.2016.5471. Epub 2016 Dec 7. Oncol Lett. 2017. PMID: 28356970 Free PMC article.
-
Optimized Longitudinal Monitoring of Stem Cell Grafts in Mouse Brain Using a Novel Bioluminescent/Near Infrared Fluorescent Fusion Reporter.Cell Transplant. 2017 Dec;26(12):1878-1889. doi: 10.1177/0963689717739718. Cell Transplant. 2017. PMID: 29390874 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

