Prospect of Stem Cells in Bone Tissue Engineering: A Review
- PMID: 26880976
- PMCID: PMC4736569
- DOI: 10.1155/2016/6180487
Prospect of Stem Cells in Bone Tissue Engineering: A Review
Abstract
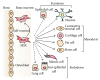
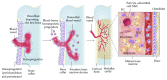
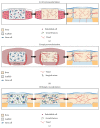
Mesenchymal stem cells (MSCs) have been the subject of many studies in recent years, ranging from basic science that looks into MSCs properties to studies that aim for developing bioengineered tissues and organs. Adult bone marrow-derived mesenchymal stem cells (BM-MSCs) have been the focus of most studies due to the inherent potential of these cells to differentiate into various cell types. Although, the discovery of induced pluripotent stem cells (iPSCs) represents a paradigm shift in our understanding of cellular differentiation. These cells are another attractive stem cell source because of their ability to be reprogramed, allowing the generation of multiple cell types from a single cell. This paper briefly covers various types of stem cell sources that have been used for tissue engineering applications, with a focus on bone regeneration. Then, an overview of some recent studies making use of MSC-seeded 3D scaffold systems for bone tissue engineering has been presented. The emphasis has been placed on the reported scaffold properties that tend to improve MSCs adhesion, proliferation, and osteogenic differentiation outcomes.
Figures
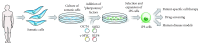



Similar articles
-
Comparative Craniofacial Bone Regeneration Capacities of Mesenchymal Stem Cells Derived from Human Neural Crest Stem Cells and Bone Marrow.ACS Biomater Sci Eng. 2021 Jan 11;7(1):207-221. doi: 10.1021/acsbiomaterials.0c00878. Epub 2020 Dec 9. ACS Biomater Sci Eng. 2021. PMID: 33455206
-
Human Induced Pluripotent Stem Cells Differentiate Into Functional Mesenchymal Stem Cells and Repair Bone Defects.Stem Cells Transl Med. 2016 Nov;5(11):1447-1460. doi: 10.5966/sctm.2015-0311. Epub 2016 Jul 11. Stem Cells Transl Med. 2016. PMID: 27400789 Free PMC article.
-
Human induced pluripotent stem cell-derived mesenchymal stem cell seeding on calcium phosphate scaffold for bone regeneration.Tissue Eng Part A. 2014 Apr;20(7-8):1295-305. doi: 10.1089/ten.TEA.2013.0211. Epub 2014 Jan 7. Tissue Eng Part A. 2014. PMID: 24279868 Free PMC article.
-
Different Sources of Mesenchymal Stem Cells for Tissue Regeneration: A Guide to Identifying the Most Favorable One in Orthopedics and Dentistry Applications.Int J Mol Sci. 2022 Jun 6;23(11):6356. doi: 10.3390/ijms23116356. Int J Mol Sci. 2022. PMID: 35683035 Free PMC article. Review.
-
Osteogenically differentiated mesenchymal stem cells and ceramics for bone tissue engineering.Expert Opin Biol Ther. 2014 Feb;14(2):197-208. doi: 10.1517/14712598.2014.866086. Epub 2013 Dec 6. Expert Opin Biol Ther. 2014. PMID: 24308323 Review.
Cited by
-
lncRNA HHIP-AS1/HHIP modulates osteogenic differentiation of BM-MSCs by regulating Hedgehog signaling pathway.Aging (Albany NY). 2022 Nov 14;14(21):8839-8855. doi: 10.18632/aging.204381. Epub 2022 Nov 14. Aging (Albany NY). 2022. PMID: 36375472 Free PMC article.
-
Mussel inspired ZIF8 microcarriers: a new approach for large-scale production of stem cells.RSC Adv. 2020 May 27;10(34):20118-20128. doi: 10.1039/d0ra04090h. eCollection 2020 May 26. RSC Adv. 2020. PMID: 35520442 Free PMC article.
-
Bioactive Sr(II)/Chitosan/Poly(ε-caprolactone) Scaffolds for Craniofacial Tissue Regeneration. In Vitro and In Vivo Behavior.Polymers (Basel). 2018 Mar 7;10(3):279. doi: 10.3390/polym10030279. Polymers (Basel). 2018. PMID: 30966314 Free PMC article.
-
Preparation and Characterization of Surface Heat Sintered Nanohydroxyapatite and Nanowhitlockite Embedded Poly (Lactic-co-glycolic Acid) Microsphere Bone Graft Scaffolds: In Vitro and in Vivo Studies.Int J Mol Sci. 2020 Jan 14;21(2):528. doi: 10.3390/ijms21020528. Int J Mol Sci. 2020. PMID: 31947689 Free PMC article.
-
Dynamic mechanical loading and growth factors influence chondrogenesis of induced pluripotent mesenchymal progenitor cells in a cartilage-mimetic hydrogel.Biomater Sci. 2019 Nov 19;7(12):5388-5403. doi: 10.1039/c9bm01081e. Biomater Sci. 2019. PMID: 31626251 Free PMC article.
References
-
- U.S. Census Bureau. Health and Nutrition. Washington, DC, USA: US Census Bureau Statistical Abstracts of the United States; 2006.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

