The Application of Human iPSCs in Neurological Diseases: From Bench to Bedside
- PMID: 26880979
- PMCID: PMC4736583
- DOI: 10.1155/2016/6484713
The Application of Human iPSCs in Neurological Diseases: From Bench to Bedside
Abstract
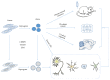
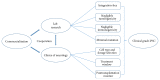
In principle, induced pluripotent stem cells (iPSCs) are generated from somatic cells by reprogramming and gaining the capacity to self-renew indefinitely as well as the ability to differentiate into cells of different lineages. Human iPSCs have absolute advantages over human embryonic stem cells (ESCs) and animal models in disease modeling, drug screening, and cell replacement therapy. Since Takahashi and Yamanaka first described in 2007 that iPSCs can be generated from human adult somatic cells by retroviral transduction of the four transcription factors, Oct3/4, Sox2, Klf4, and c-Myc, disease specific iPSC lines have sprung up worldwide like bamboo shoots after a spring rain, making iPSC one of the hottest and fastest moving topics in modern science. The craze for iPSCs has spread throughout main branches of clinical medicine, covering neurology, hematology, cardiology, endocrinology, hepatology, ophthalmology, and so on. Here in this paper, we will focus on the clinical application of human iPSCs in disease modeling, drug screening, and cell replacement therapy for neurological diseases.
Figures
Similar articles
-
Generation of two induced pluripotent stem cells lines from a Mucopolysaccharydosis IIIB (MPSIIIB) patient.Stem Cell Res. 2018 Dec;33:180-184. doi: 10.1016/j.scr.2018.10.019. Epub 2018 Nov 1. Stem Cell Res. 2018. PMID: 30408744
-
Generation of induced pluripotent stem cells with high efficiency from human embryonic renal cortical cells.Am J Transl Res. 2016 Nov 15;8(11):4982-4993. eCollection 2016. Am J Transl Res. 2016. PMID: 27904699 Free PMC article.
-
Induced pluripotent stem cells from goat fibroblasts.Mol Reprod Dev. 2013 Dec;80(12):1009-17. doi: 10.1002/mrd.22266. Epub 2013 Nov 27. Mol Reprod Dev. 2013. PMID: 24123501
-
Reverse engineering human neurodegenerative disease using pluripotent stem cell technology.Brain Res. 2016 May 1;1638(Pt A):30-41. doi: 10.1016/j.brainres.2015.09.023. Epub 2015 Sep 28. Brain Res. 2016. PMID: 26423934 Free PMC article. Review.
-
Clinical Application of Induced Pluripotent Stem Cells in Cardiovascular Medicine.Cardiology. 2015;131(4):236-44. doi: 10.1159/000381280. Epub 2015 May 12. Cardiology. 2015. PMID: 25969168 Review.
Cited by
-
The translational potential of human induced pluripotent stem cells for clinical neurology : The translational potential of hiPSCs in neurology.Cell Biol Toxicol. 2017 Apr;33(2):129-144. doi: 10.1007/s10565-016-9372-7. Epub 2016 Dec 3. Cell Biol Toxicol. 2017. PMID: 27915387 Free PMC article. Review.
-
On the Road from Phenotypic Plasticity to Stem Cell Therapy.J Neurosci. 2021 Jun 23;41(25):5331-5337. doi: 10.1523/JNEUROSCI.0340-21.2021. Epub 2021 May 6. J Neurosci. 2021. PMID: 33958488 Free PMC article.
-
Reprogramming of mouse fibroblasts into neural lineage cells using biomaterials.Bioimpacts. 2018;8(2):129-138. doi: 10.15171/bi.2018.15. Epub 2018 Jan 10. Bioimpacts. 2018. PMID: 29977834 Free PMC article.
-
ASXL3 Is a Novel Pluripotency Factor in Human Respiratory Epithelial Cells and a Potential Therapeutic Target in Small Cell Lung Cancer.Cancer Res. 2017 Nov 15;77(22):6267-6281. doi: 10.1158/0008-5472.CAN-17-0570. Epub 2017 Sep 21. Cancer Res. 2017. PMID: 28935813 Free PMC article.
-
Tissue Engineering and Regenerative Medicine: Perspectives and Challenges.MedComm (2020). 2025 Apr 24;6(5):e70192. doi: 10.1002/mco2.70192. eCollection 2025 May. MedComm (2020). 2025. PMID: 40290901 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

