Endogenous and Synthetic Cannabinoids as Therapeutics in Retinal Disease
- PMID: 26881135
- PMCID: PMC4736800
- DOI: 10.1155/2016/8373020
Endogenous and Synthetic Cannabinoids as Therapeutics in Retinal Disease
Abstract
The functional significance of cannabinoids in ocular physiology and disease has been reported some decades ago. In the early 1970s, subjects who smoked Cannabis sativa developed lower intraocular pressure (IOP). This led to the isolation of phytocannabinoids from this plant and the study of their therapeutic effects in glaucoma. The main treatment of this disease to date involves the administration of drugs mediating either the decrease of aqueous humour synthesis or the increase of its outflow and thus reduces IOP. However, the reduction of IOP is not sufficient to prevent visual field loss. Retinal diseases, such as glaucoma and diabetic retinopathy, have been defined as neurodegenerative diseases and characterized by ischemia-induced excitotoxicity and loss of retinal neurons. Therefore, new therapeutic strategies must be applied in order to target retinal cell death, reduction of visual acuity, and blindness. The aim of the present review is to address the neuroprotective and therapeutic potential of cannabinoids in retinal disease.
Figures

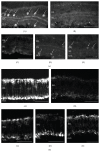
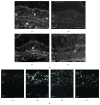
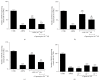
References
-
- Merritt J. C., Perry D. D., Russell D. N., Jones B. F. Topical delta 9-tetrahydrocannabinol and aqueous dynamics in glaucoma. Journal of Clinical Pharmacology. 1981;21(8-9, supplement):467S–471S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

