Cell line and patient-derived xenograft models reveal elevated CDCP1 as a target in high-grade serous ovarian cancer
- PMID: 26882065
- PMCID: PMC4815773
- DOI: 10.1038/bjc.2015.471
Cell line and patient-derived xenograft models reveal elevated CDCP1 as a target in high-grade serous ovarian cancer
Abstract
Background: Development of targeted therapies for high-grade serous ovarian cancer (HGSC) remains challenging, as contributing molecular pathways are poorly defined or expressed heterogeneously. CUB-domain containing protein 1 (CDCP1) is a cell-surface protein elevated in lung, colorectal, pancreas, renal and clear cell ovarian cancer.
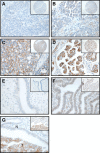
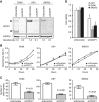
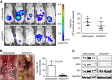
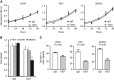
Methods: CUB-domain containing protein 1 was examined by immunohistochemistry in HGSC and fallopian tube. The impact of targeting CDCP1 on cell growth and migration in vitro, and intraperitoneal xenograft growth in mice was examined. Three patient-derived xenograft (PDX) mouse models were developed and characterised for CDCP1 expression. The effect of a monoclonal anti-CDCP1 antibody on PDX growth was examined. Src activation was assessed by western blot analysis.
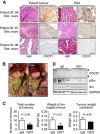
Results: Elevated CDCP1 was observed in 77% of HGSC cases. Silencing of CDCP1 reduced migration and non-adherent cell growth in vitro and tumour burden in vivo. Expression of CDCP1 in patient samples was maintained in PDX models. Antibody blockade of CDCP1 significantly reduced growth of an HGSC PDX. The CDCP1-mediated activation of Src was observed in cultured cells and mouse xenografts.
Conclusions: CUB-domain containing protein 1 is over-expressed by the majority of HGSCs. In vitro and mouse model data indicate that CDCP1 has a role in HGSC and that it can be targeted to inhibit progression of this cancer.
Conflict of interest statement
JDH is an inventor on a patent describing CDCP1 as an anti-cancer target.
Figures

References
-
- Adams MN, Harrington BS, He Y, Davies CM, Wallace SJ, Chetty NP, Crandon AJ, Oliveira NB, Shannon CM, Coward JI, Lumley JW, Perrin LC, Armes JE, Hooper JD (2015) EGF inhibits constitutive internalization and palmitoylation-dependent degradation of membrane-spanning procancer CDCP1 promoting its availability on the cell surface. Oncogene 34(11): 1375–1383. - PubMed
-
- Al-Hussaini M, Stockman A, Foster H, McCluggage WG (2004) WT-1 assists in distinguishing ovarian from uterine serous carcinoma and in distinguishing between serous and endometrioid ovarian carcinoma. Histopathology 44(2): 109–115. - PubMed
-
- Armes JE, Davies CM, Wallace S, Taheri T, Perrin LC, Autelitano DJ (2013) AGR2 expression in ovarian tumours: a potential biomarker for endometrioid and mucinous differentiation. Pathology 45(1): 49–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

