Non-thermal atmospheric plasma induces ROS-independent cell death in U373MG glioma cells and augments the cytotoxicity of temozolomide
- PMID: 26882067
- PMCID: PMC4815779
- DOI: 10.1038/bjc.2016.12
Non-thermal atmospheric plasma induces ROS-independent cell death in U373MG glioma cells and augments the cytotoxicity of temozolomide
Abstract
Background: Non-thermal atmospheric plasma (NTAP) is an ionised gas produced under high voltage that can generate short-lived chemically active species and induce a cytotoxic insult in cancer cells. Cell-specific resistance to NTAP-mediated cytotoxicity has been reported in the literature. The aim of this study was to determine whether resistance against NTAP could be overcome using the human glioma cell line U373MG.
Methods: Non-thermal atmospheric plasma was generated using a Dielectric Barrier Device (DBD) system with a maximum voltage output of 120 kV at 50 Hz. The viability of U373MG GBM cells and HeLa cervical carcinoma cells was determined using morphology, flow cytometry and cytotoxicity assays. Fluorescent probes and inhibitors were used to determine the mechanisms of cytotoxicity of cells exposed to the plasma field. Combinational therapy with temozolomide (TMZ) and multi-dose treatments were explored as mechanisms to overcome resistance to NTAP.
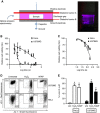
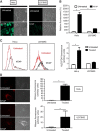
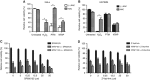
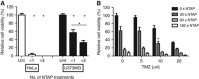
Results: Non-thermal atmospheric plasma decreased cell viability in a dose (time)-dependent manner. U373MG cells were shown to be resistant to NTAP treatment when compared with HeLa cells, and the levels of intracellular reactive oxygen species (ROS) quantified in U373MG cells were much lower than in HeLa cells following exposure to the plasma field. Reactive oxygen species inhibitor N-acetyl cysteine (NAC) only alleviated the cytotoxic effects in HeLa cells and not in the relatively NTAP-resistant cell line U373MG. Longer exposures to NTAP induced a cell death independent of ROS, JNK and caspases in U373MG. The relative resistance of U373MG cells to NTAP could be overcome when used in combination with low concentrations of the GBM chemotherapy TMZ or exposure to multiple doses.
Conclusions: For the very first time, we report that NTAP induces an ROS-, JNK- and caspase-independent mechanism of cell death in the U373MG GBM cell line that can be greatly enhanced when used in combination with low doses of TMZ. Further refinement of the technology may facilitate localised activation of cytotoxicity against GBM when used in combination with new and existing chemotherapeutic regimens.
Figures
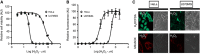
References
-
- Adamson C, Kanu OO, Mehta AI, Di C, Lin N, Mattox AK, Bigner DD (2009) Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs 18: 1061–1083. - PubMed
-
- Candolfi M, Curtin JF, Nichols WS, Muhammad AG, King GD, Pluhar GE, McNiel EA, Ohlfest JR, Freese AB, Moore PF, Lerner J, Lowenstein PR, Castro MG (2007) Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J Neurooncol 85: 133–148. - PMC - PubMed
-
- Carrabba G, Mukhopadhyay D, Guha A (2009) Aberrant signalling complexes in GBMs: prognostic and therapeutic implications. Glioblastoma: Molecular Mechanisms of Pathogenesis and Current Therapeutic Strategies. Springer Science and Business Media: New York, NY, USA, pp 95–130.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

