Inflammatory cytokine and chemokine profiles are associated with patient outcome and the hyperadrenergic state following acute brain injury
- PMID: 26883121
- PMCID: PMC4754875
- DOI: 10.1186/s12974-016-0500-3
Inflammatory cytokine and chemokine profiles are associated with patient outcome and the hyperadrenergic state following acute brain injury
Abstract
Background: Traumatic brain injury (TBI) elicits intense sympathetic nervous system (SNS) activation with profuse catecholamine secretion. The resultant hyperadrenergic state is linked to immunomodulation both within the brain and systemically. Dysregulated inflammation post-TBI exacerbates secondary brain injury and contributes to unfavorable patient outcomes including death. The aim of this study was to characterize the early dynamic profile of circulating inflammatory cytokines/chemokines in patients admitted for moderate-to-severe TBI, to examine interrelationships between these mediators and catecholamines, as well as clinical indices of injury severity and neurological outcome.
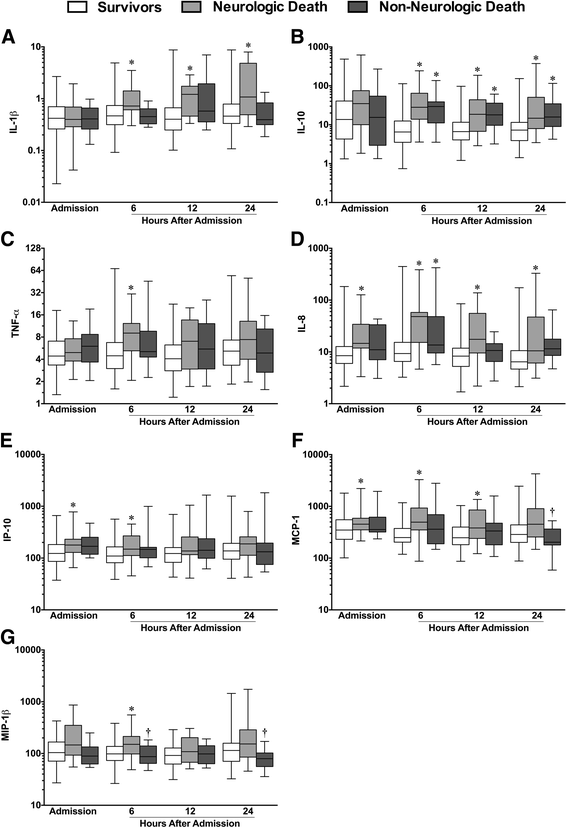
Methods: Blood was sampled from 166 isolated TBI patients (aged 45 ± 20.3 years; 74.7 % male) on admission, 6-, 12-, and 24-h post-injury and from healthy controls (N = 21). Plasma cytokine [interleukin (IL)-1β, -2, -4, -5, -10, -12p70, -13, tumor necrosis factor (TNF)-α, interferon (IFN)-γ] and chemokine [IL-8, eotaxin, eotaxin-3, IFN-γ-induced protein (IP)-10, monocyte chemoattractant protein (MCP)-1, -4, macrophage-derived chemokine (MDC), macrophage inflammatory protein (MIP)-1β, thymus activation regulated chemokine (TARC)] concentrations were analyzed using high-sensitivity electrochemiluminescence multiplex immunoassays. Plasma catecholamines [epinephrine (Epi), norepinephrine (NE)] were measured by immunoassay. Neurological outcome at 6 months was assessed using the extended Glasgow outcome scale (GOSE) dichotomized as good (>4) or poor (≤4) outcomes.
Results: Patients showed altered levels of IL-10 and all chemokines assayed relative to controls. Significant differences in a number of markers were evident between moderate and severe TBI cohorts. Elevated IL-8, IL-10, and TNF-α, as well as alterations in 8 of 9 chemokines, were associated with poor outcome at 6 months. Notably, a positive association was found between Epi and IL-1β, IL-10, Eotaxin, IL-8, and MCP-1. NE was positively associated with IL-1β, IL-10, TNF-α, eotaxin, IL-8, IP-10, and MCP-1.
Conclusions: Our results provide further evidence that exaggerated SNS activation acutely after isolated TBI in humans may contribute to harmful peripheral inflammatory cytokine/chemokine dysregulation. These findings are consistent with a potentially beneficial role for therapies aimed at modulating the inflammatory response and hyperadrenergic state acutely post-injury.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

