Evaluating the effectiveness of selected community-level interventions on key maternal, child health, and prevention of mother-to-child transmission of HIV outcomes in three countries (the ACCLAIM Project): a study protocol for a randomized controlled trial
- PMID: 26883307
- PMCID: PMC4754877
- DOI: 10.1186/s13063-016-1202-y
Evaluating the effectiveness of selected community-level interventions on key maternal, child health, and prevention of mother-to-child transmission of HIV outcomes in three countries (the ACCLAIM Project): a study protocol for a randomized controlled trial
Abstract
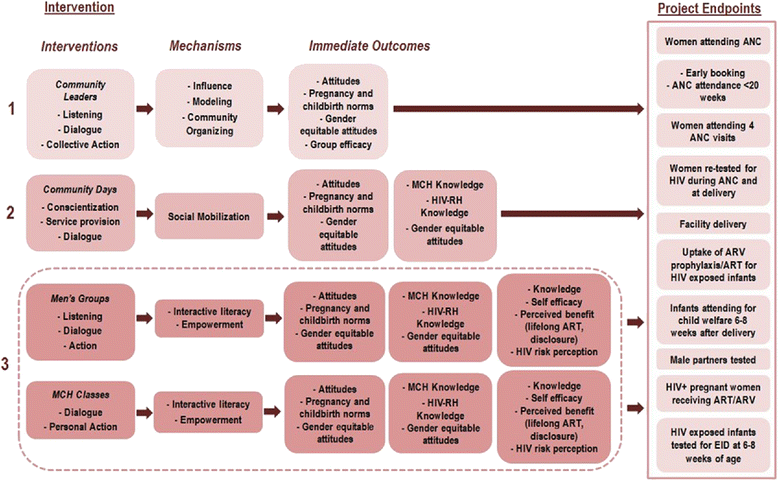
Background: Efforts to scale up and improve programs for prevention of mother-to-child transmission of HIV (PMTCT) have focused primarily at the health facility level, and limited attention has been paid to defining an effective set of community interventions to improve demand and uptake of services and retention. Many barriers to PMTCT are also barriers to pregnancy, childbirth, and postnatal care faced by mothers regardless of HIV status. Demand for maternal and child health (MCH) and PMTCT services can be limited by critical social, cultural, and structural barriers. Yet, rigorous evaluation has shown limited evidence of effectiveness of multilevel community-wide interventions aimed at improving MCH and HIV outcomes for pregnant women living with HIV. We propose to assess the effect of a package of multilevel community interventions: a social learning and action component, community dialogues, and peer-led discussion groups, on the demand for, uptake of, and retention of HIV positive pregnant/postpartum women in MCH/PMTCT services.
Methods/design: This study will undertake a three-arm randomized trial in Swaziland, Uganda, and Zimbabwe. Districts/regions (n = 9) with 45 PMTCT-implementing health facilities and their catchment areas (populations 7,300-27,500) will be randomly allocated to three intervention arms: 1) community leader engagement, 2) community leader engagement with community days, or 3) community leader engagement with community days and male and female community peer groups. The primary study outcome is HIV exposed infants (HEIs) returning to the health facility within 2 months for early infant diagnosis (EID) of HIV. Secondary study outcomes include gestational age of women attending for first antenatal care, male partners tested for HIV, and HEIs receiving nevirapine prophylaxis at birth. Changes in community knowledge, attitudes, practices, and beliefs on MCH/PMTCT will be assessed through household surveys.
Discussion: Implementation of the protocol necessitated changes in the original study design. We purposively selected facilities in the districts/regions though originally the study clusters were to be randomly selected. Lifelong antiretroviral therapy for all HIV positive pregnant and lactating women, Option B+, was implemented in the three countries during the study period, with the potential for a differential impact by study arm. Implementation however, was rapidly done across the districts/regions, so that there is unlikely be this potential confounding. We developed a system of monitoring and documentation of potential confounding activities or actions, and these data will be incorporated into analyses at the conclusion of the project. Strengthens of the study are that it tests multilevel interventions, utilizes program as well as study specific and individual data, and it is conducted under "real conditions" leading to more robust findings. Limitations of the protocol include the lack of a true control arm and inadequate control for the potential effect of Option B+, such as the intensification of messages as the importance of early ANC and male partner testing.
Trial registration: ClinicalTrials.gov (study ID: NCT01971710) Protocol version 5, 30 July 2013, registered 13 August 2013.
Figures
References
-
- Joint United Nations Programme on HIV/AIDS. Global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. Geneva: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2011.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical