Reprogramming of blood cells into induced pluripotent stem cells as a new cell source for cartilage repair
- PMID: 26883322
- PMCID: PMC4756426
- DOI: 10.1186/s13287-016-0290-7
Reprogramming of blood cells into induced pluripotent stem cells as a new cell source for cartilage repair
Abstract
Background: An attempt was made to reprogram peripheral blood cells into human induced pluripotent stem cell (hiPSCs) as a new cell source for cartilage repair.
Methods: We generated chondrogenic lineage from human peripheral blood via hiPSCs using an integration-free method. Peripheral blood cells were either obtained from a human blood bank or freshly collected from volunteers. After transforming peripheral blood cells into iPSCs, the newly derived iPSCs were further characterized through karyotype analysis, pluripotency gene expression and cell differentiation ability. iPSCs were differentiated through multiple steps, including embryoid body formation, hiPSC-mesenchymal stem cell (MSC)-like cell expansion, and chondrogenic induction for 21 days. Chondrocyte phenotype was then assessed by morphological, histological and biochemical analysis, as well as the chondrogenic expression.
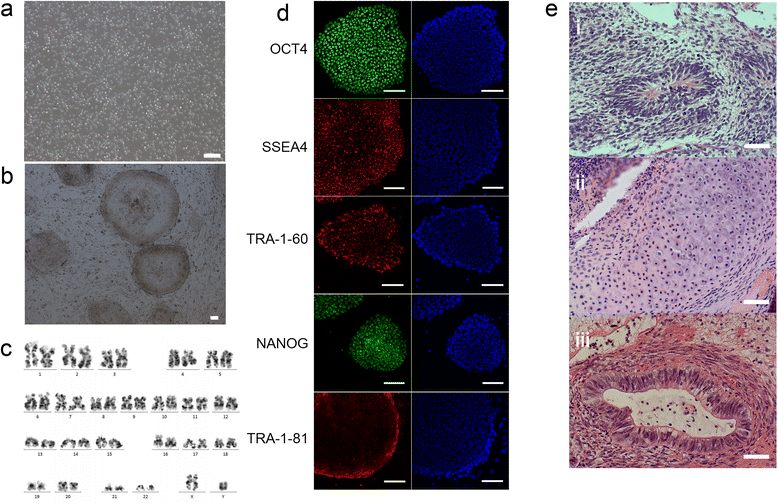
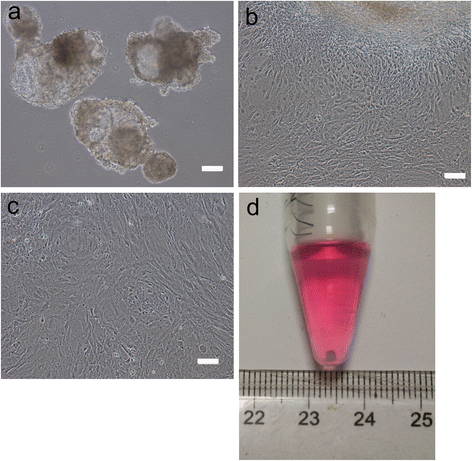
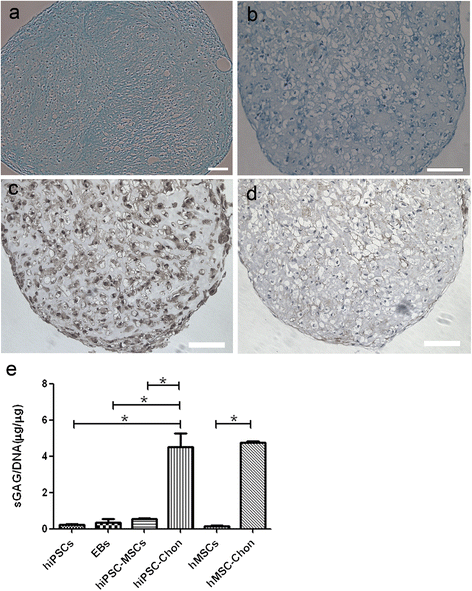
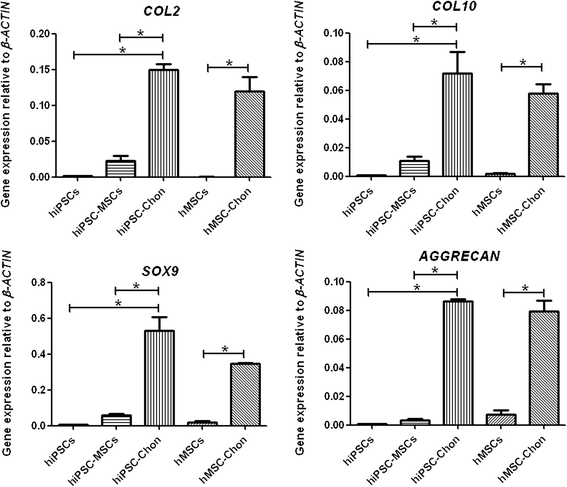
Results: hiPSCs derived from peripheral blood cells were successfully generated, and were characterized by fluorescent immunostaining of pluripotent markers and teratoma formation in vivo. Flow cytometric analysis showed that MSC markers CD73 and CD105 were present in monolayer cultured hiPSC-MSC-like cells. Both alcian blue and toluidine blue staining of hiPSC-MSC-chondrogenic pellets showed as positive. Immunohistochemistry of collagen II and X staining of the pellets were also positive. The sulfated glycosaminoglycan content was significantly increased, and the expression levels of the chondrogenic markers COL2, COL10, COL9 and AGGRECAN were significantly higher in chondrogenic pellets than in undifferentiated cells. These results indicated that peripheral blood cells could be a potential source for differentiation into chondrogenic lineage in vitro via generation of mesenchymal progenitor cells.
Conclusions: This study supports the potential applications of utilizing peripheral blood cells in generating seed cells for cartilage regenerative medicine in a patient-specific and cost-effective approach.
Figures

Similar articles
-
Cord blood cell-derived iPSCs as a new candidate for chondrogenic differentiation and cartilage regeneration.Stem Cell Res Ther. 2017 Jan 28;8(1):16. doi: 10.1186/s13287-017-0477-6. Stem Cell Res Ther. 2017. PMID: 28129782 Free PMC article.
-
Differentiating Chondrocytes from Peripheral Blood-derived Human Induced Pluripotent Stem Cells.J Vis Exp. 2017 Jul 18;(125):55722. doi: 10.3791/55722. J Vis Exp. 2017. PMID: 28745632 Free PMC article.
-
Chondrogenic Pellet Formation from Cord Blood-derived Induced Pluripotent Stem Cells.J Vis Exp. 2017 Jun 19;(124):55988. doi: 10.3791/55988. J Vis Exp. 2017. PMID: 28654049 Free PMC article.
-
Cellular reprogramming for clinical cartilage repair.Cell Biol Toxicol. 2017 Aug;33(4):329-349. doi: 10.1007/s10565-017-9382-0. Epub 2017 Jan 31. Cell Biol Toxicol. 2017. PMID: 28144824 Free PMC article. Review.
-
[Cartilage regeneration using cell reprogramming technologies].Clin Calcium. 2013 Nov;23(11):1641-8. Clin Calcium. 2013. PMID: 24162605 Review. Japanese.
Cited by
-
A novel PDCD10 gene mutation in cerebral cavernous malformations: a case report and review of the literature.J Pain Res. 2019 Apr 1;12:1127-1132. doi: 10.2147/JPR.S190317. eCollection 2019. J Pain Res. 2019. PMID: 31114296 Free PMC article.
-
Current Therapeutic Strategies for Stem Cell-Based Cartilage Regeneration.Stem Cells Int. 2018 Mar 25;2018:8490489. doi: 10.1155/2018/8490489. eCollection 2018. Stem Cells Int. 2018. PMID: 29765426 Free PMC article. Review.
-
Stem Cells for Cartilage Repair: Preclinical Studies and Insights in Translational Animal Models and Outcome Measures.Stem Cells Int. 2018 Feb 5;2018:9079538. doi: 10.1155/2018/9079538. eCollection 2018. Stem Cells Int. 2018. PMID: 29535784 Free PMC article. Review.
-
The Role of Extracellular Matrix Expression, ERK1/2 Signaling and Cell Cohesiveness for Cartilage Yield from iPSCs.Int J Mol Sci. 2019 Sep 2;20(17):4295. doi: 10.3390/ijms20174295. Int J Mol Sci. 2019. PMID: 31480758 Free PMC article.
-
IGF-1 Gene Transfer to Human Synovial MSCs Promotes Their Chondrogenic Differentiation Potential without Induction of the Hypertrophic Phenotype.Stem Cells Int. 2017;2017:5804147. doi: 10.1155/2017/5804147. Epub 2017 Jun 27. Stem Cells Int. 2017. PMID: 28740513 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

