The fibrosis-cell death axis in heart failure
- PMID: 26883434
- PMCID: PMC4762920
- DOI: 10.1007/s10741-016-9536-9
The fibrosis-cell death axis in heart failure
Abstract
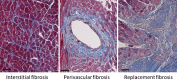
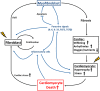
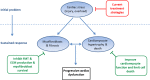
Cardiac stress can induce morphological, structural and functional changes of the heart, referred to as cardiac remodeling. Myocardial infarction or sustained overload as a result of pathological causes such as hypertension or valve insufficiency may result in progressive remodeling and finally lead to heart failure (HF). Whereas pathological and physiological (exercise, pregnancy) overload both stimulate cardiomyocyte growth (hypertrophy), only pathological remodeling is characterized by increased deposition of extracellular matrix proteins, termed fibrosis, and loss of cardiomyocytes by necrosis, apoptosis and/or phagocytosis. HF is strongly associated with age, and cardiomyocyte loss and fibrosis are typical signs of the aging heart. Fibrosis results in stiffening of the heart, conductivity problems and reduced oxygen diffusion, and is associated with diminished ventricular function and arrhythmias. As a consequence, the workload of cardiomyocytes in the fibrotic heart is further augmented, whereas the physiological environment is becoming less favorable. This causes additional cardiomyocyte death and replacement of lost cardiomyocytes by fibrotic material, generating a vicious cycle of further decline of cardiac function. Breaking this fibrosis-cell death axis could halt further pathological and age-related cardiac regression and potentially reverse remodeling. In this review, we will describe the interaction between cardiac fibrosis, cardiomyocyte hypertrophy and cell death, and discuss potential strategies for tackling progressive cardiac remodeling and HF.
Keywords: Cardiomyocyte; Cell death; Fibroblast; Fibrosis; Heart failure; Hypertrophy; Myofibroblast; TGFβ.
Figures
References
-
- Rockey DC, Bell PD, Hill JA. Fibrosis: a common pathway to organ injury and failure. N Engl J Med. 2015;12:1138–1149. - PubMed
-
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;8:803–869. - PubMed
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;16:e147–e239. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

