Protective Effect of Chronic Schistosomiasis in Baboons Coinfected with Schistosoma mansoni and Plasmodium knowlesi
- PMID: 26883586
- PMCID: PMC4862699
- DOI: 10.1128/IAI.00490-15
Protective Effect of Chronic Schistosomiasis in Baboons Coinfected with Schistosoma mansoni and Plasmodium knowlesi
Abstract
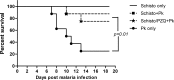
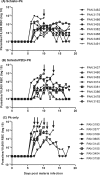
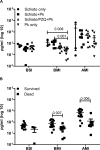
Malaria and schistosomiasis coinfections are common, and chronic schistosomiasis has been implicated in affecting the severity of acute malaria. However, whether it enhances or attenuates malaria has been controversial due the lack of appropriately controlled human studies and relevant animal models. To examine this interaction, we conducted a randomized controlled study using the baboon (Papio anubis) to analyze the effect of chronic schistosomiasis on severe malaria. Two groups of baboons (n = 8 each) and a schistosomiasis control group (n = 3) were infected with 500 Schistosoma mansoni cercariae. At 14 and 15 weeks postinfection, one group was given praziquantel to treat schistosomiasis infection. Four weeks later, the two groups plus a new malaria control group (n = 8) were intravenously inoculated with 10(5) Plasmodium knowlesi parasites and monitored daily for development of severe malaria. A total of 81% of baboons exposed to chronic S. mansoni infection with or without praziquantel treatment survived malaria, compared to only 25% of animals infected with P. knowlesi only (P = 0.01). Schistosome-infected animals also had significantly lower parasite burdens (P = 0.004) than the baboons in the P. knowlesi-only group and were protected from severe anemia. Coinfection was associated with increased spontaneous production of interleukin-6 (IL-6), suggesting an enhanced innate immune response, whereas animals infected with P. knowlesi alone failed to develop mitogen-driven tumor necrosis factor alpha and IL-10, indicating the inability to generate adequate protective and balancing immunoregulatory responses. These results indicate that chronic S. mansoni attenuates the severity of P. knowlesi coinfection in baboons by mechanisms that may enhance innate immunity to malaria.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures






Similar articles
-
Acquired Clinical Immunity to Malaria in Nonhuman Primates Coinfected with Schistosoma and Plasmodium Parasites.Infect Immun. 2022 Feb 17;90(2):e0046421. doi: 10.1128/IAI.00464-21. Epub 2021 Dec 6. Infect Immun. 2022. PMID: 34871040 Free PMC article.
-
Chronic whipworm infection exacerbates Schistosoma mansoni egg-induced hepatopathology in non-human primates.Parasit Vectors. 2020 Feb 28;13(1):109. doi: 10.1186/s13071-020-3980-z. Parasit Vectors. 2020. PMID: 32111243 Free PMC article.
-
Experimental infection of the olive baboon (Paplio anubis) with Plasmodium knowlesi: severe disease accompanied by cerebral involvement.Am J Trop Med Hyg. 2003 Aug;69(2):188-94. Am J Trop Med Hyg. 2003. PMID: 13677374
-
Coinfection of Schistosoma Species with Hepatitis B or Hepatitis C Viruses.Adv Parasitol. 2016;91:111-231. doi: 10.1016/bs.apar.2015.12.003. Epub 2016 Feb 5. Adv Parasitol. 2016. PMID: 27015949 Review.
-
The baboon as a non-human primate model of human schistosome infection.Parasitol Today. 1999 Dec;15(12):478-82. doi: 10.1016/s0169-4758(99)01569-0. Parasitol Today. 1999. PMID: 10557147 Review.
Cited by
-
Infection against infection: parasite antagonism against parasites, viruses and bacteria.Infect Dis Poverty. 2019 Jun 15;8(1):49. doi: 10.1186/s40249-019-0560-6. Infect Dis Poverty. 2019. PMID: 31200765 Free PMC article. Review.
-
Beyond schistosomiasis: unraveling co-infections and altered immunity.Clin Microbiol Rev. 2024 Mar 14;37(1):e0009823. doi: 10.1128/cmr.00098-23. Epub 2024 Feb 6. Clin Microbiol Rev. 2024. PMID: 38319102 Free PMC article. Review.
-
Application of microphysiological systems to unravel the mechanisms of schistosomiasis egg extravasation.Front Cell Infect Microbiol. 2025 Feb 18;15:1521265. doi: 10.3389/fcimb.2025.1521265. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40041145 Free PMC article. Review.
-
The Road to Elimination: Current State of Schistosomiasis Research and Progress Towards the End Game.Front Immunol. 2022 May 3;13:846108. doi: 10.3389/fimmu.2022.846108. eCollection 2022. Front Immunol. 2022. PMID: 35592327 Free PMC article. Review.
-
Avian malaria-mediated population decline of a widespread iconic bird species.R Soc Open Sci. 2019 Jul 17;6(7):182197. doi: 10.1098/rsos.182197. eCollection 2019 Jul. R Soc Open Sci. 2019. PMID: 31417708 Free PMC article.
References
-
- World Health Organization. 2013. World malaria report–2013. World Health Organization, Geneva, Switzerland.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

