Bicoid gradient formation and function in the Drosophila pre-syncytial blastoderm
- PMID: 26883601
- PMCID: PMC4786422
- DOI: 10.7554/eLife.13222
Bicoid gradient formation and function in the Drosophila pre-syncytial blastoderm
Erratum in
-
Correction: Bicoid gradient formation and function in the Drosophila pre-syncytial blastoderm.Elife. 2017 Mar 15;6:e26811. doi: 10.7554/eLife.26811. Elife. 2017. PMID: 28296637 Free PMC article. No abstract available.
Abstract
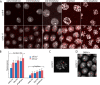
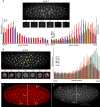
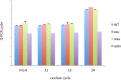
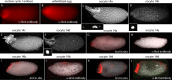
Bicoid (Bcd) protein distributes in a concentration gradient that organizes the anterior/posterior axis of the Drosophila embryo. It has been understood that bcd RNA is sequestered at the anterior pole during oogenesis, is not translated until fertilization, and produces a protein gradient that functions in the syncytial blastoderm after 9-10 nuclear divisions. However, technical issues limited the sensitivity of analysis of pre-syncytial blastoderm embryos and precluded studies of oocytes after stage 13. We developed methods to analyze stage 14 oocytes and pre-syncytial blastoderm embryos, and found that stage 14 oocytes make Bcd protein, that bcd RNA and Bcd protein distribute in matching concentration gradients in the interior of nuclear cycle 2-6 embryos, and that Bcd regulation of target gene expression is apparent at nuclear cycle 7, two cycles prior to syncytial blastoderm. We discuss the implications for the generation and function of the Bcd gradient.
Keywords: d. melanogaster; bicoid; developmental biology; drosophila; embryo; krüppel; oocyte; stem cells.
Conflict of interest statement
The author declares that no competing interests exist.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

