Proof-of Concept that an Acute Trophic Factors Intervention After Spinal Cord Injury Provides an Adequate Niche for Neuroprotection, Recruitment of Nestin-Expressing Progenitors and Regeneration
- PMID: 26883642
- PMCID: PMC5352162
- DOI: 10.1007/s11064-016-1850-z
Proof-of Concept that an Acute Trophic Factors Intervention After Spinal Cord Injury Provides an Adequate Niche for Neuroprotection, Recruitment of Nestin-Expressing Progenitors and Regeneration
Erratum in
-
Erratum to: Proof-of Concept that an Acute Trophic Factors Intervention After Spinal Cord Injury Provides an Adequate Niche for Neuroprotection, Recruitment of Nestin-Expressing Progenitors and Regeneration.Neurochem Res. 2016 Jul;41(7):1844. doi: 10.1007/s11064-016-1925-x. Neurochem Res. 2016. PMID: 27180188 Free PMC article. No abstract available.
Abstract
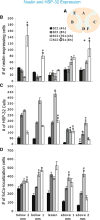
Trophic factor treatment has been shown to improve the recovery of brain and spinal cord injury (SCI). In this study, we examined the effects of TSC1 (a combination of insulin-like growth factor 1 and transferrin) 4 and 8 h after SCI at the thoracic segment level (T12) in nestin-GFP transgenic mice. TSC1 treatment for 4 and 8 h increased the number of nestin-expressing cells around the lesion site and prevented Wallerian degeneration. Treatment with TSC1 for 4 h significantly increased heat shock protein (HSP)-32 and HSP-70 expression 1 and 2 mm from lesion site (both, caudal and rostral). Conversely, the number of HSP-32 positive cells decreased after an 8-h TSC1 treatment, although it was still higher than in both, non-treated SCI and intact spinal cord animals. Furthermore, TSC1 increased NG2 expressing cell numbers and preserved most axons intact, facilitating remyelination and repair. These results support our hypothesis that TSC1 is an effective treatment for cell and tissue neuroprotection after SCI. An early intervention is crucial to prevent secondary damage of the injured SC and, in particular, to prevent Wallerian degeneration.
Keywords: Heat shock proteins; IGF-1; Myelin; Oligodendrocytes; Transferrin; Wallerian degeneration.
Figures






Similar articles
-
Mixed primary culture and clonal analysis provide evidence that NG2 proteoglycan-expressing cells after spinal cord injury are glial progenitors.Dev Neurobiol. 2007 Jun;67(7):860-74. doi: 10.1002/dneu.20369. Dev Neurobiol. 2007. PMID: 17506499
-
Erythropoietin-mediated preservation of the white matter in rat spinal cord injury.Neuroscience. 2007 Feb 9;144(3):865-77. doi: 10.1016/j.neuroscience.2006.10.023. Epub 2006 Dec 4. Neuroscience. 2007. PMID: 17141961
-
Characterization and therapeutic evaluation of a Nestin⁺ CNP⁺ NG2⁺ cell population on mouse spinal cord injury.Exp Neurol. 2015 Jul;269:28-42. doi: 10.1016/j.expneurol.2015.03.030. Epub 2015 Apr 7. Exp Neurol. 2015. PMID: 25862288
-
Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury.Prog Brain Res. 2002;137:257-73. doi: 10.1016/s0079-6123(02)37020-1. Prog Brain Res. 2002. PMID: 12440372 Review.
-
Myelin status and oligodendrocyte lineage cells over time after spinal cord injury: What do we know and what still needs to be unwrapped?Glia. 2019 Nov;67(11):2178-2202. doi: 10.1002/glia.23702. Epub 2019 Aug 24. Glia. 2019. PMID: 31444938 Free PMC article. Review.
Cited by
-
Transgenic models for investigating the nervous system: Currently available neurofluorescent reporters and potential neuronal markers.Biochim Biophys Acta Gen Subj. 2020 Jul;1864(7):129595. doi: 10.1016/j.bbagen.2020.129595. Epub 2020 Mar 12. Biochim Biophys Acta Gen Subj. 2020. PMID: 32173376 Free PMC article. Review.
-
Role and Therapeutic Potential of Melatonin in the Central Nervous System and Cancers.Cancers (Basel). 2020 Jun 13;12(6):1567. doi: 10.3390/cancers12061567. Cancers (Basel). 2020. PMID: 32545820 Free PMC article. Review.
-
Executive Dysfunction Early Postnatal Biomarkers among Children Born Extremely Preterm.J Neuroimmune Pharmacol. 2019 Jun;14(2):188-199. doi: 10.1007/s11481-018-9804-7. Epub 2018 Sep 6. J Neuroimmune Pharmacol. 2019. PMID: 30191383 Free PMC article.
-
Experimental Treatments for Spinal Cord Injury: A Systematic Review and Meta-Analysis.Cells. 2022 Oct 28;11(21):3409. doi: 10.3390/cells11213409. Cells. 2022. PMID: 36359804 Free PMC article.
References
-
- Nelson E, Gertz SD, Rennels ML, Ducker TB, Blaumanis OR. Spinal cord injury. The role of vascular damage in the pathogenesis of central hemorrhagic necrosis. Arch Neurol. 1997;34:332–333. - PubMed
-
- Lu J, Ashwell KW, Waite P. Advances in secondary spinal cord injury: role of apoptosis. Spine. 2000;25(14):1859–1866. - PubMed
-
- Chang H, Wo JP, Tzeng SF. Neuroprotection of glial cell line-derived neurotrophic factor in damaged spinal cords following contusive injury. J Neuro Res. 2002;69:397–405. - PubMed
-
- McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417–425. - PubMed
-
- Beattie MS, Bresnahan JC, Komon J, et al. Endogenous repair after spinal cord contusion injuries in the rat. Exp Neurol. 1997;148(2):453–463. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

