Solithromycin Pharmacokinetics in Plasma and Dried Blood Spots and Safety in Adolescents
- PMID: 26883693
- PMCID: PMC4808196
- DOI: 10.1128/AAC.02561-15
Solithromycin Pharmacokinetics in Plasma and Dried Blood Spots and Safety in Adolescents
Abstract
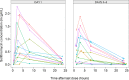
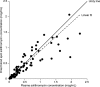
We assessed the pharmacokinetics and safety of solithromycin, a fluoroketolide antibiotic, in a phase 1, open-label, multicenter study of 13 adolescents with suspected or confirmed bacterial infections. On days 3 to 5, the mean (standard deviation) maximum plasma concentration and area under the concentration versus time curve from 0 to 24 h were 0.74 μg/ml (0.61 μg/ml) and 9.28 μg · h/ml (6.30 μg · h/ml), respectively. The exposure and safety in this small cohort of adolescents were comparable to those for adults. (This study has been registered at ClinicalTrials.gov under registration no. NCT01966055.).
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- US Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013... Accessed 19 October, 2015.
-
- Gonzalez D, Melloni C, Poindexter BB, Yogev R, Atz AM, Sullivan JE, Mendley SR, Delmore P, Delinsky A, Zimmerman K, Lewandowski A, Harper B, Lewis KC, Benjamin DK Jr, Cohen-Wokowiez M, Best Pharmaceuticals for Children Act—Pediatric Trials Network Administrative Core Committee. 2015. Simultaneous determination of trimethoprim and sulfamethoxazole in dried plasma and urine spots. Bioanalysis 7:1137–1149. doi:10.4155/bio.15.38. - DOI - PMC - PubMed
-
- Parsons TL, Marzinke MA, Hoang T, Bliven-Sizemore E, Weiner M, Mac Kenzie WR, Dorman SE, Dooley KE. 2014. Quantification of rifapentine, a potent anti-tuberculosis drug, from dried blood spot samples using liquid chromatographic-tandem mass spectrometric analysis. Antimicrob Agents Chemother 58:6747–6757. doi:10.1128/AAC.03607-14. - DOI - PMC - PubMed
-
- Still JG, Schranz J, Degenhardt TP, Scott D, Fernandes P, Gutierrez MJ, Clark K. 2011. Pharmacokinetics of solithromycin (CEM-101) after single or multiple oral doses and effects of food on single-dose bioavailability in healthy adult subjects. Antimicrob Agents Chemother 55:1997–2003. doi:10.1128/AAC.01429-10. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HHSN275201000001Z/HD/NICHD NIH HHS/United States
- K24 HD058735/HD/NICHD NIH HHS/United States
- R01 HD081044/HD/NICHD NIH HHS/United States
- HHSN272201500001C/AI/NIAID NIH HHS/United States
- U01 FD004858/FD/FDA HHS/United States
- HHSN275201000003C/HD/NICHD NIH HHS/United States
- HHSN275201000003I/HD/NICHD NIH HHS/United States
- UL1 TR001117/TR/NCATS NIH HHS/United States
- HHSN272201500006C/AI/NIAID NIH HHS/United States
- HHSN272201300017C/AI/NIAID NIH HHS/United States
- HHSN272201300017I/AI/NIAID NIH HHS/United States
- K23 HD083465/HD/NICHD NIH HHS/United States
- HHSN272201500001G/AI/NIAID NIH HHS/United States
- R01 HD076676/HD/NICHD NIH HHS/United States
- HHSN275201000001G/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

