N-Terminal Acetylation-Targeted N-End Rule Proteolytic System: The Ac/N-End Rule Pathway
- PMID: 26883906
- PMCID: PMC4794598
- DOI: 10.14348/molcells.2016.2329
N-Terminal Acetylation-Targeted N-End Rule Proteolytic System: The Ac/N-End Rule Pathway
Abstract
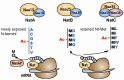
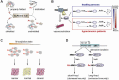
Although Nα-terminal acetylation (Nt-acetylation) is a pervasive protein modification in eukaryotes, its general functions in a majority of proteins are poorly understood. In 2010, it was discovered that Nt-acetylation creates a specific protein degradation signal that is targeted by a new class of the N-end rule proteolytic system, called the Ac/N-end rule pathway. Here, we review recent advances in our understanding of the mechanism and biological functions of the Ac/N-end rule pathway, and its crosstalk with the Arg/N-end rule pathway (the classical N-end rule pathway).
Keywords: N-end rule; N-terminal acetylation; degron; proteolysis; ubiquitin.
Figures

References
-
- Aksnes H., Drazic A., Arnesen T. (Hyper)tension release by N-terminal acetylation. Trends Biochem. Sci. 2015a;40:422–424. - PubMed
-
- Aksnes H., Hole K., Arnesen T. Molecular, cellular, and physiological significance of N-terminal acetylation. Int. Rev. Cell Mol. Biol. 2015b;316:267–305. - PubMed
-
- Aksnes H., Van Damme P., Goris M., Starheim K.K., Marie M., Stove S.I., Hoel C., Kalvik T.V., Hole K., Glomnes N., et al. An organellar nalpha-acetyltransferase, naa60, acetylates cytosolic N termini of transmembrane proteins and maintains Golgi integrity. Cell Rep. 2015c;10:1362–1374. - PubMed
-
- Arnesen T., Van Damme P., Polevoda B., Helsens K., Evjenth R., Colaert N., Varhaug J.E., Vandekerckhove J., Lillehaug J.R., Sherman F., et al. Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans. Proc. Natl. Acad. Sci. USA. 2009;106:8157–8162. - PMC - PubMed
-
- Arnesen T., Starheim K.K., Van Damme P., Evjenth R., Dinh H., Betts M.J., Ryningen A., Vandekerckhove J., Gevaert K., Anderson D. The chaperone-like protein HYPK acts together with NatA in cotranslational N-terminal acetylation and prevention of Huntingtin aggregation. Mol. Cell. Biol. 2010;30:1898–1909. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

