Tracking the immunopathological response to Pseudomonas aeruginosa during respiratory infections
- PMID: 26883959
- PMCID: PMC4756310
- DOI: 10.1038/srep21465
Tracking the immunopathological response to Pseudomonas aeruginosa during respiratory infections
Abstract
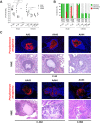
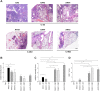
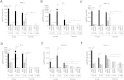
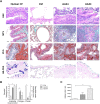
Repeated cycles of infections, caused mainly by Pseudomonas aeruginosa, combined with a robust host immune response and tissue injury, determine the course and outcome of cystic fibrosis (CF) lung disease. As the disease progresses, P. aeruginosa adapts to the host modifying dramatically its phenotype; however, it remains unclear whether and how bacterial adaptive variants and their persistence influence the pathogenesis and disease development. Using in vitro and murine models of infection, we showed that P. aeruginosa CF-adaptive variants shaped the innate immune response favoring their persistence. Next, we refined a murine model of chronic pneumonia extending P. aeruginosa infection up to three months. In this model, including CFTR-deficient mice, we unveil that the P. aeruginosa persistence lead to CF hallmarks of airway remodelling and fibrosis, including epithelial hyperplasia and structure degeneration, goblet cell metaplasia, collagen deposition, elastin degradation and several additional markers of tissue damage. This murine model of P. aeruginosa chronic infection, reproducing CF lung pathology, will be instrumental to identify novel molecular targets and test newly tailored molecules inhibiting chronic inflammation and tissue damage processes in pre-clinical studies.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

