DNA and Protein Requirements for Substrate Conformational Changes Necessary for Human Flap Endonuclease-1-catalyzed Reaction
- PMID: 26884332
- PMCID: PMC4825025
- DOI: 10.1074/jbc.M115.698993
DNA and Protein Requirements for Substrate Conformational Changes Necessary for Human Flap Endonuclease-1-catalyzed Reaction
Abstract
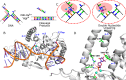
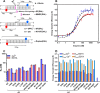
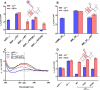
Human flap endonuclease-1 (hFEN1) catalyzes the essential removal of single-stranded flaps arising at DNA junctions during replication and repair processes. hFEN1 biological function must be precisely controlled, and consequently, the protein relies on a combination of protein and substrate conformational changes as a prerequisite for reaction. These include substrate bending at the duplex-duplex junction and transfer of unpaired reacting duplex end into the active site. When present, 5'-flaps are thought to thread under the helical cap, limiting reaction to flaps with free 5'-terminiin vivo Here we monitored DNA bending by FRET and DNA unpairing using 2-aminopurine exciton pair CD to determine the DNA and protein requirements for these substrate conformational changes. Binding of DNA to hFEN1 in a bent conformation occurred independently of 5'-flap accommodation and did not require active site metal ions or the presence of conserved active site residues. More stringent requirements exist for transfer of the substrate to the active site. Placement of the scissile phosphate diester in the active site required the presence of divalent metal ions, a free 5'-flap (if present), a Watson-Crick base pair at the terminus of the reacting duplex, and the intact secondary structure of the enzyme helical cap. Optimal positioning of the scissile phosphate additionally required active site conserved residues Tyr(40), Asp(181), and Arg(100)and a reacting duplex 5'-phosphate. These studies suggest a FEN1 reaction mechanism where junctions are bound and 5'-flaps are threaded (when present), and finally the substrate is transferred onto active site metals initiating cleavage.
Keywords: DNA endonuclease; DNA repair; DNA replication; DNA-protein interaction; circular dichroism (CD); fluorescence resonance energy transfer (FRET); nucleic acid enzymology.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


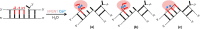
References
-
- Finger L. D., Atack J. M., Tsutakawa S., Classen S., Tainer J., Grasby J., and Shen B. (2012) The wonders of flap endonucleases: structure, function, mechanism, and regulation. In The Eukaryotic Replisome: A Guide to Protein Structure and Function (MacNeill S., ed) pp. 301–326, Springer-Verlag, New York Inc., New York - PMC - PubMed
-
- Tomlinson C. G., Atack J. M., Chapados B., Tainer J. A., and Grasby J. A. (2010) Substrate recognition and catalysis by flap endonucleases and related enzymes. Biochem. Soc. Trans. 38, 433–437 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

