Dynamic Remodeling of the Magnetosome Membrane Is Triggered by the Initiation of Biomineralization
- PMID: 26884433
- PMCID: PMC4791847
- DOI: 10.1128/mBio.01898-15
Dynamic Remodeling of the Magnetosome Membrane Is Triggered by the Initiation of Biomineralization
Abstract
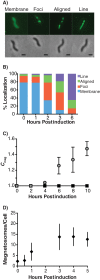
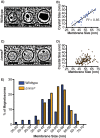
Magnetotactic bacteria produce chains of membrane-bound organelles that direct the biomineralization of magnetic nanoparticles. These magnetosome compartments are a model for studying the biogenesis and subcellular organization of bacterial organelles. Previous studies have suggested that discrete gene products build and assemble magnetosomes in a stepwise fashion. Here, using an inducible system, we show that the stages of magnetosome formation are highly dynamic and interconnected. During de novo formation, magnetosomes first organize into discontinuous chain fragments that are subsequently connected by the bacterial actin-like protein MamK. We also find that magnetosome membranes are not uniform in size and can grow in a biomineralization-dependent manner. In the absence of biomineralization, magnetosome membranes stall at a diameter of ~50 nm. Those that have initiated biomineralization then expand to significantly larger sizes and accommodate mature magnetic particles. We speculate that such a biomineralization-dependent checkpoint for membrane growth establishes the appropriate conditions within the magnetosome to ensure successful nucleation and growth of magnetic particles.
Importance: Magnetotactic bacteria make magnetic nanoparticles inside membrane-bound organelles called magnetosomes; however, it is unclear how the magnetosome membrane controls the biomineralization that occurs within this bacterial organelle. We placed magnetosome formation under inducible control in Magnetospirillum magneticum AMB-1 and used electron cryo-tomography to capture magnetosomes in their near-native state as they form de novo. An inducible system provided the key evidence that magnetosome membranes grow continuously unless they have not properly initiated biomineralization. Our finding that the size of a bacterial organelle impacts its biochemical function is a fundamental advance that impacts our perception of organelle formation and can inform future attempts aimed at creating designer magnetic particles.
Copyright © 2016 Cornejo et al.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

