The matricellular protein CCN1 enhances TGF-β1/SMAD3-dependent profibrotic signaling in fibroblasts and contributes to fibrogenic responses to lung injury
- PMID: 26884454
- PMCID: PMC4871800
- DOI: 10.1096/fj.201500173
The matricellular protein CCN1 enhances TGF-β1/SMAD3-dependent profibrotic signaling in fibroblasts and contributes to fibrogenic responses to lung injury
Abstract
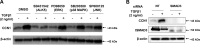
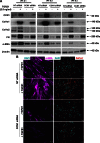
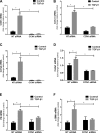
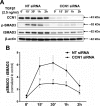
Matricellular proteins mediate pleiotropic effects during tissue injury and repair. CCN1 is a matricellular protein that has been implicated in angiogenesis, inflammation, and wound repair. In this study, we identified CCN1 as a gene that is differentially up-regulated in alveolar mesenchymal cells of human subjects with rapidly progressive idiopathic pulmonary fibrosis (IPF). Elevated levels of CCN1 mRNA were confirmed in lung tissues of IPF subjects undergoing lung transplantation, and CCN1 protein was predominantly localized to fibroblastic foci. CCN1 expression in ex vivo IPF lung fibroblasts correlated with gene expression of the extracellular matrix proteins, collagen (Col)1a1, Col1a2, and fibronectin as well as the myofibroblast marker, α-smooth muscle actin. RNA interference (RNAi)-mediated knockdown of CCN1 down-regulated the constitutive expression of these profibrotic genes in IPF fibroblasts. TGF-β1, a known mediator of tissue fibrogenesis, induces gene and protein expression of CCN1 via a mothers against decapentaplegic homolog 3 (SMAD3)-dependent mechanism. Importantly, endogenous CCN1 potentiates TGF-β1-induced SMAD3 activation and induction of profibrotic genes, supporting a positive feedback loop leading to myofibroblast activation. In vivo RNAi-mediated silencing of CCN1 attenuates fibrogenic responses to bleomycin-induced lung injury. These studies support previously unrecognized, cooperative interaction between the CCN1 matricellular protein and canonical TGF-β1/SMAD3 signaling that promotes lung fibrosis.-Kurundkar, A. R., Kurundkar, D., Rangarajan, S., Locy, M. L., Zhou, Y., Liu, R.-M., Zmijewski, J., Thannickal, V. J. The matricellular protein CCN1 enhances TGF-β1/SMAD3-dependent profibrotic signaling in fibroblasts and contributes to fibrogenic responses to lung injury.
Keywords: idiopathic pulmonary fibrosis; matrix remodeling; myofibroblasts; wound repair.
© FASEB.
Figures




References
-
- Bornstein P., Sage E. H. (2002) Matricellular proteins: extracellular modulators of cell function. Curr. Opin. Cell Biol. 14, 608–616 - PubMed
-
- Kyriakides T. R., Bornstein P. (2003) Matricellular proteins as modulators of wound healing and the foreign body response. Thromb. Haemost. 90, 986–992 - PubMed
-
- Kubota S., Takigawa M. (2007) CCN family proteins and angiogenesis: from embryo to adulthood. Angiogenesis 10, 1–11 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

